CRACKING Crude oil and Cracking Lesson objective To

CRACKING
Crude oil and Cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why the boiling points of different alkanes are different
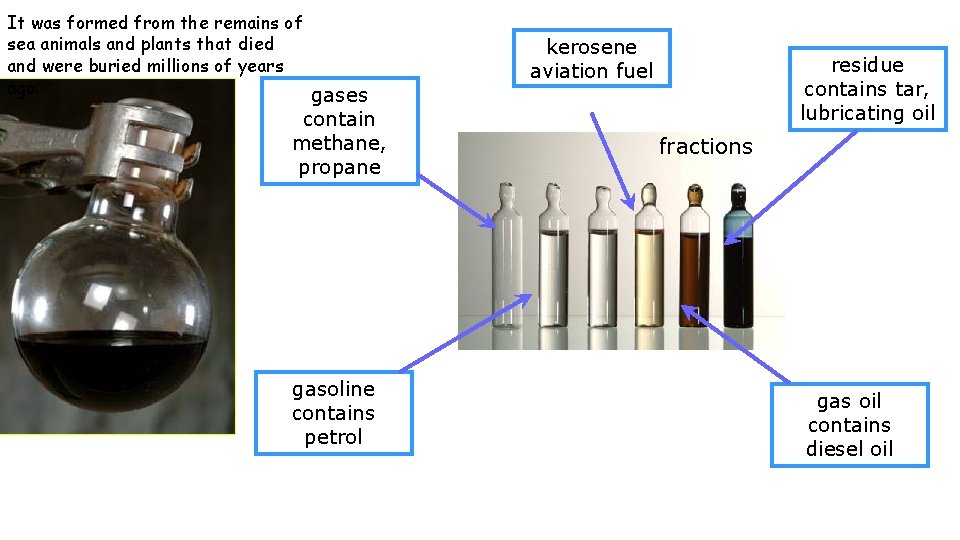
It was formed from the remains of sea animals and plants that died and were buried millions of years ago. gases contain methane, propane gasoline contains petrol kerosene aviation fuel residue contains tar, lubricating oil fractions gas oil contains diesel oil
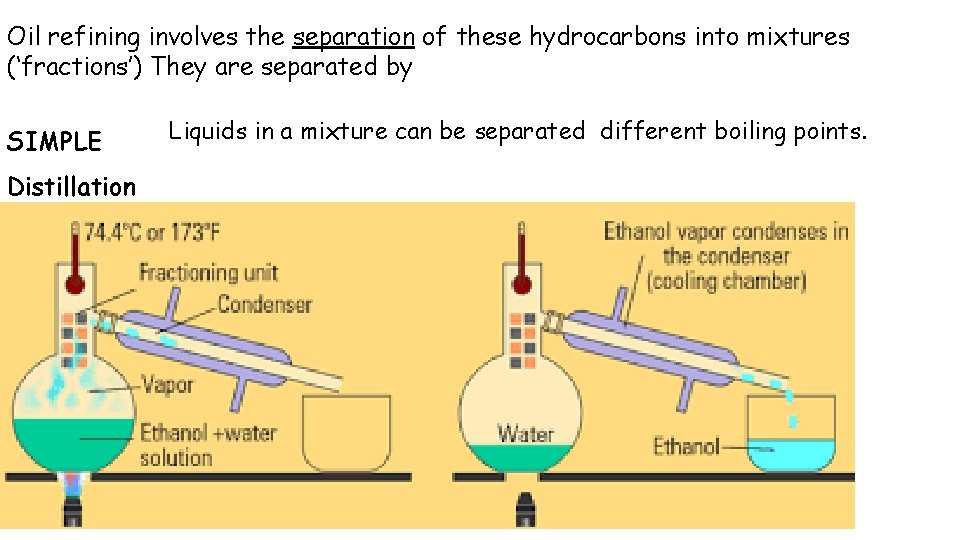
Oil refining involves the separation of these hydrocarbons into mixtures (‘fractions’) They are separated by SIMPLE Distillation 4 Liquids in a mixture can be separated different boiling points.
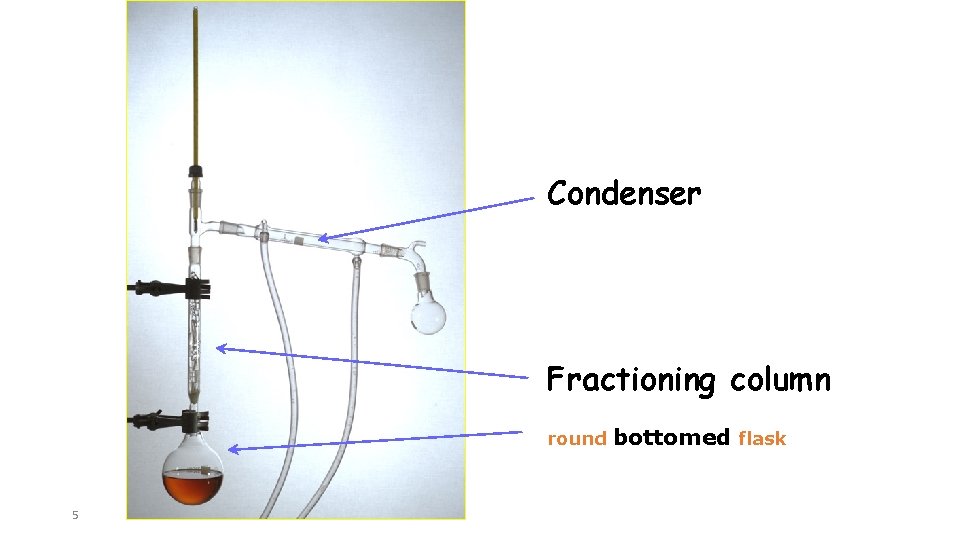
Condenser Fractioning column round 5 bottomed flask
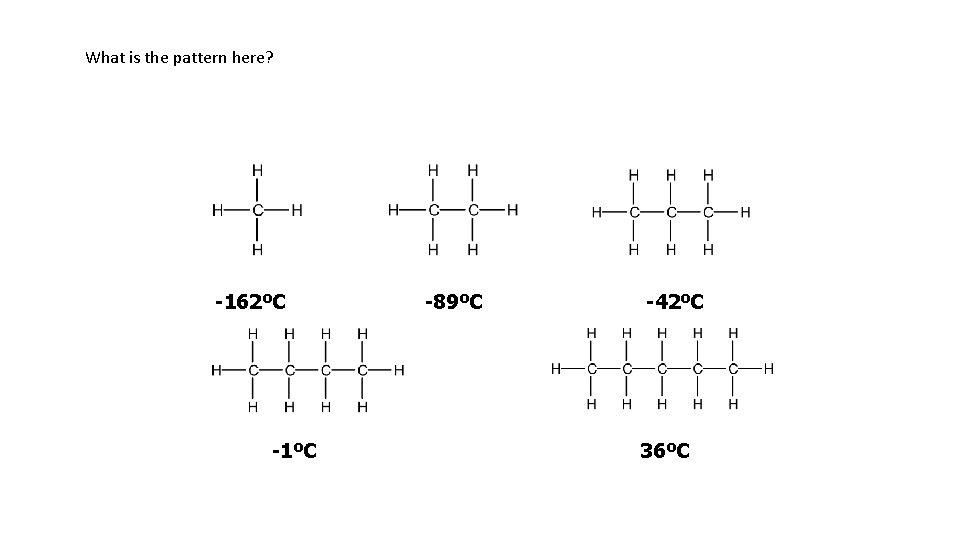
What is the pattern here? -162ºC -1ºC -89ºC -42ºC 36ºC
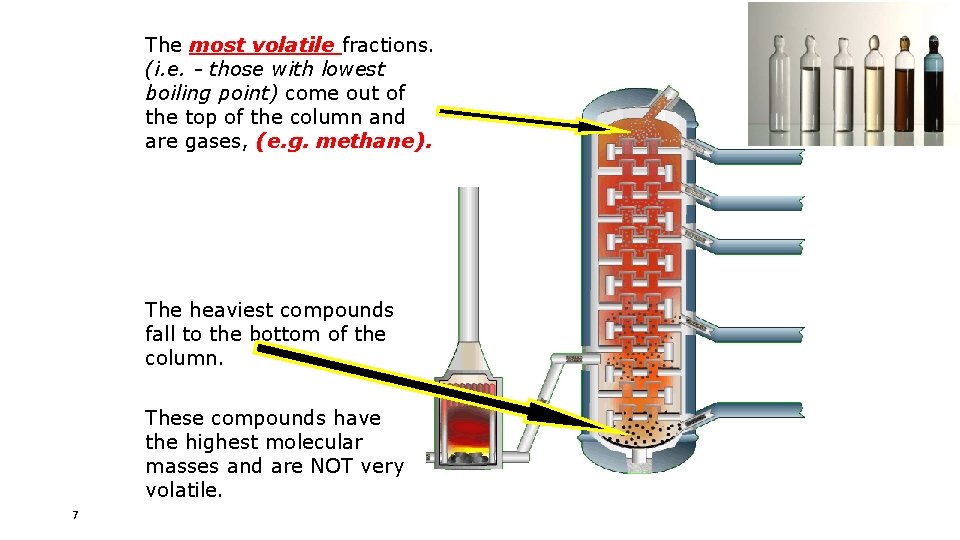
The most volatile fractions. (i. e. - those with lowest boiling point) come out of the top of the column and are gases, (e. g. methane). The heaviest compounds fall to the bottom of the column. These compounds have the highest molecular masses and are NOT very volatile. 7
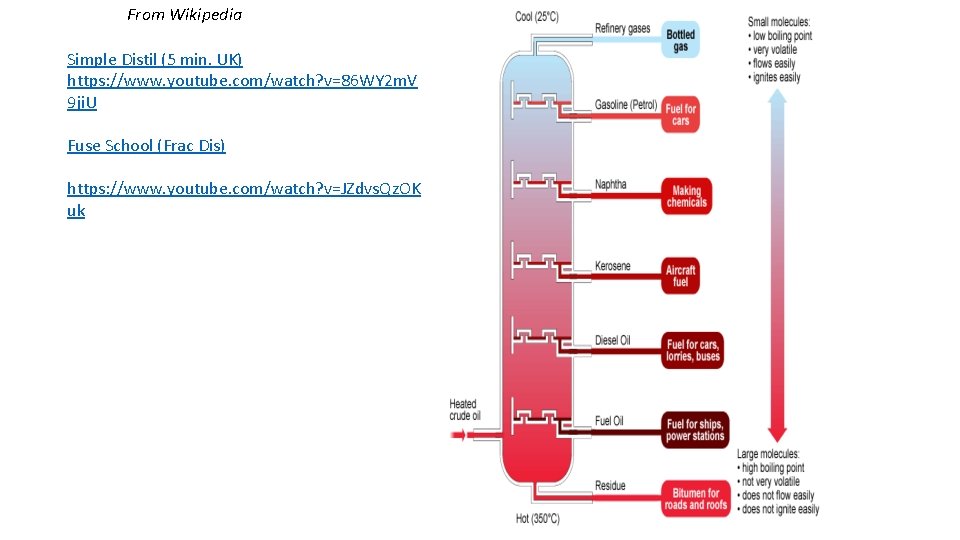
From Wikipedia Simple Distil (5 min. UK) https: //www. youtube. com/watch? v=86 WY 2 m. V 9 ji. U Fuse School (Frac Dis) https: //www. youtube. com/watch? v=JZdvs. Qz. OK uk

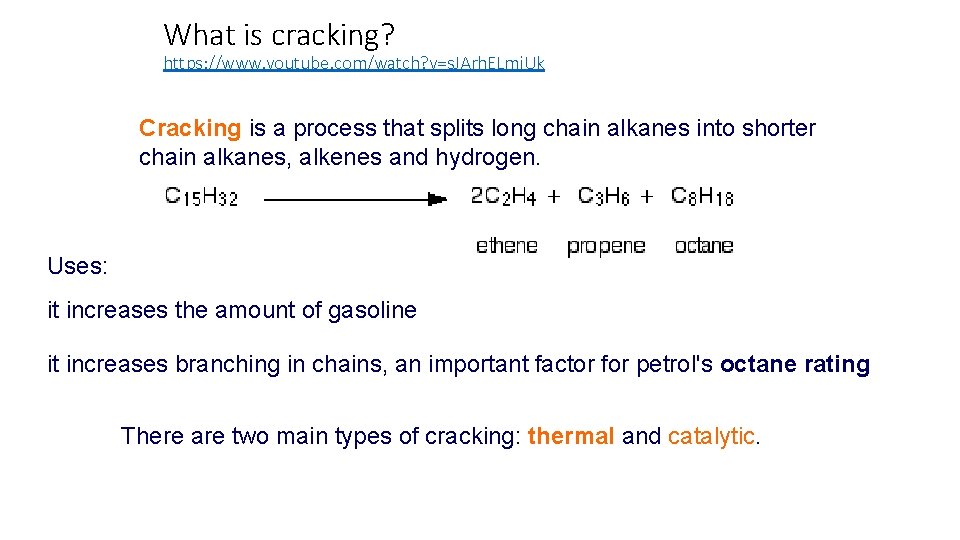
What is cracking? https: //www. youtube. com/watch? v=s. JArh. ELmi. Uk Cracking is a process that splits long chain alkanes into shorter chain alkanes, alkenes and hydrogen. Uses: it increases the amount of gasoline it increases branching in chains, an important factor for petrol's octane rating There are two main types of cracking: thermal and catalytic.
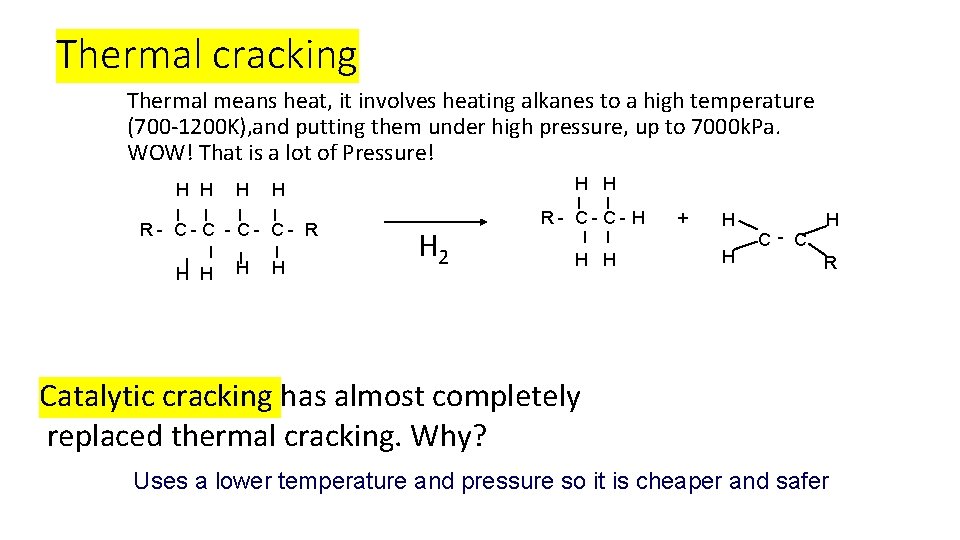
Thermal cracking Thermal means heat, it involves heating alkanes to a high temperature (700 -1200 K), and putting them under high pressure, up to 7000 k. Pa. WOW! That is a lot of Pressure! H H H R- C- C- R H H H H 2 R- C- C- H H H + H H C- C H R Catalytic cracking has almost completely replaced thermal cracking. Why? Uses a lower temperature and pressure so it is cheaper and safer
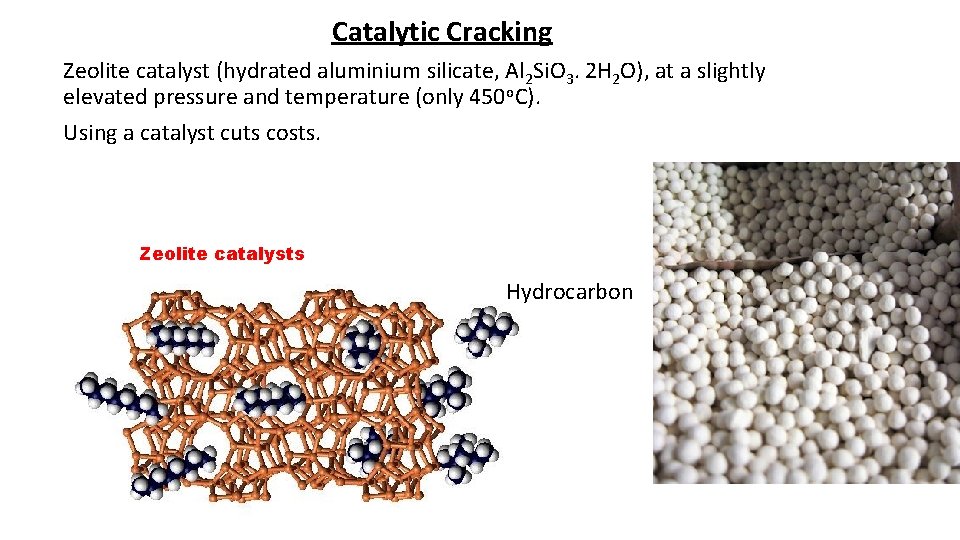
Catalytic Cracking Zeolite catalyst (hydrated aluminium silicate, Al 2 Si. O 3. 2 H 2 O), at a slightly elevated pressure and temperature (only 450 o. C). Using a catalyst cuts costs. Zeolite catalysts Hydrocarbon
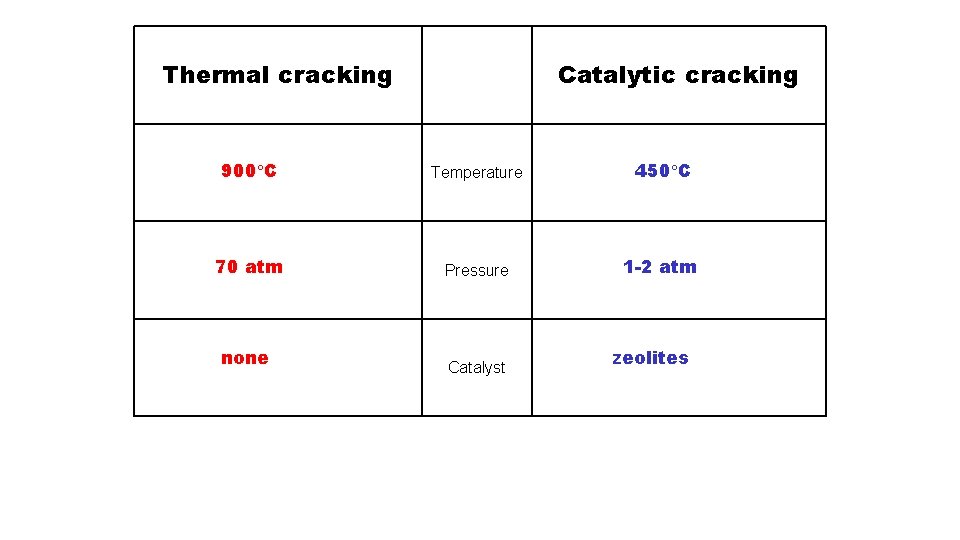
Thermal cracking Catalytic cracking 900 C Temperature 450 C 70 atm Pressure 1 -2 atm none Catalyst zeolites
- Slides: 13