CP Biology Chapter 2 The Chemistry of Life
CP Biology Chapter 2 The Chemistry of Life
Why is chemistry important in the study of biology? Chemicals make up ALL matter – living and nonliving. • All life processes are chemical reactions. • Chemical signals between cells regulate metabolism – enzymes, hormones • Chemical signals in the environment – Attract a mate; attract pollinators – Scare away predators – Find food
ELEMENTS, ATOMS, AND MOLECULES There are 92 naturally occurring elements – Only 4 make up most of the human body – CARBON – HYDROGEN (C H O N) OXYGEN NITROGEN
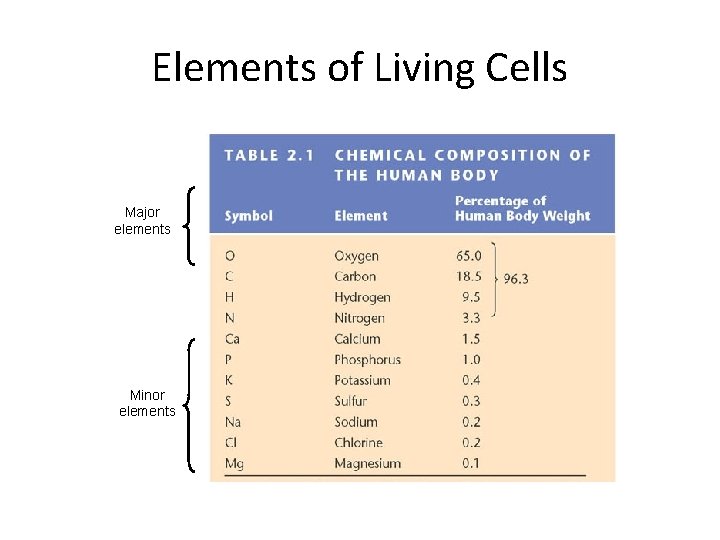
Elements of Living Cells Major elements Minor elements

Forming Compounds Two or more elements combined in a fixed ratio IONIC BONDING
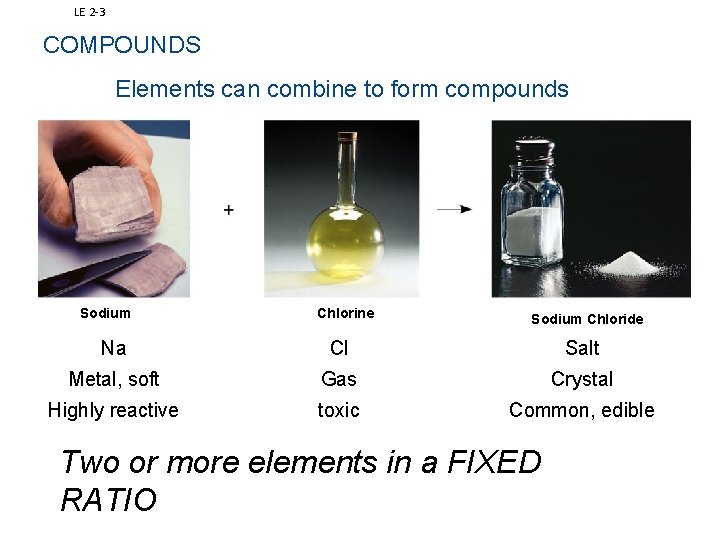
LE 2 3 COMPOUNDS Elements can combine to form compounds Sodium Chlorine Sodium Chloride Na Cl Salt Metal, soft Gas Crystal Highly reactive toxic Common, edible Two or more elements in a FIXED RATIO
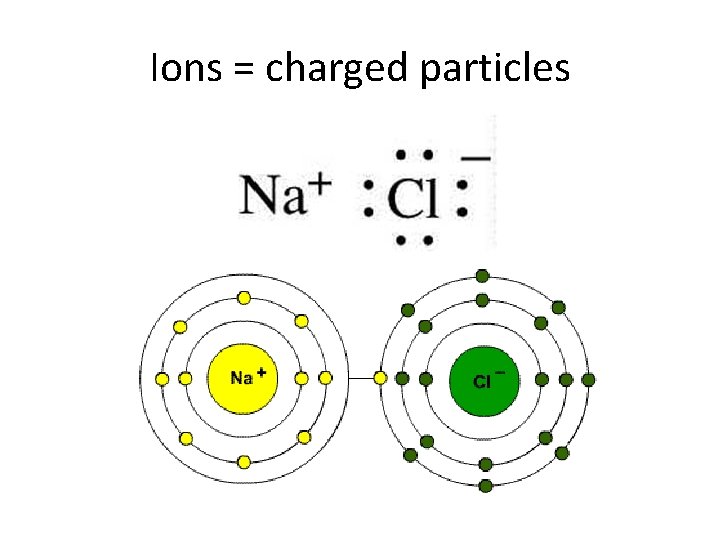
Ions = charged particles
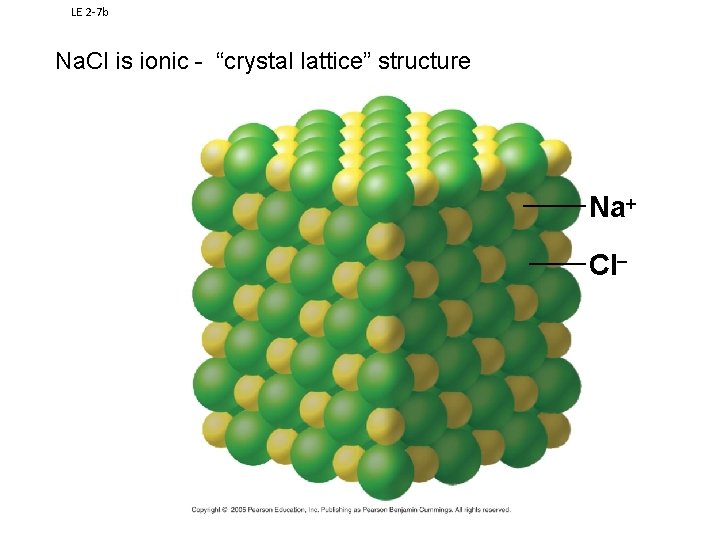
LE 2 7 b Na. Cl is ionic - “crystal lattice” structure Na+ Cl-
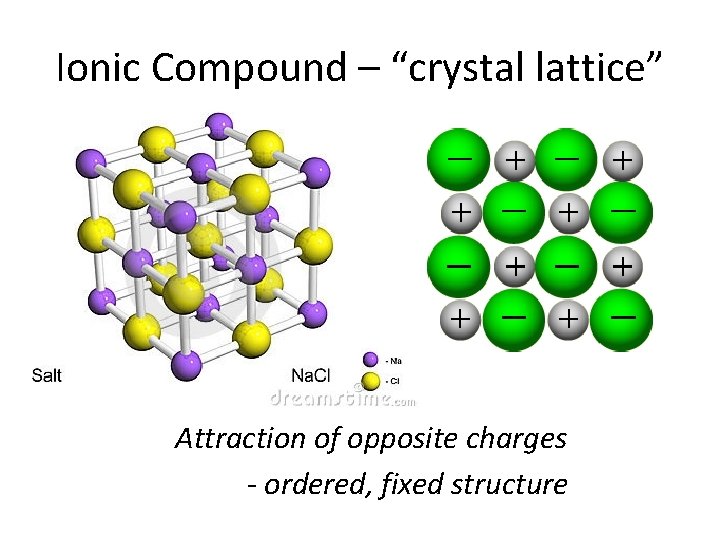
Ionic Compound – “crystal lattice” Attraction of opposite charges - ordered, fixed structure
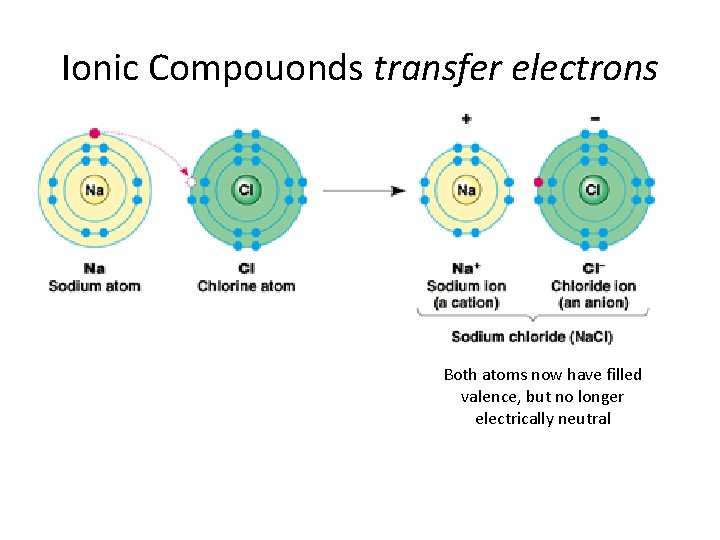
Ionic Compouonds transfer electrons Both atoms now have filled valence, but no longer electrically neutral
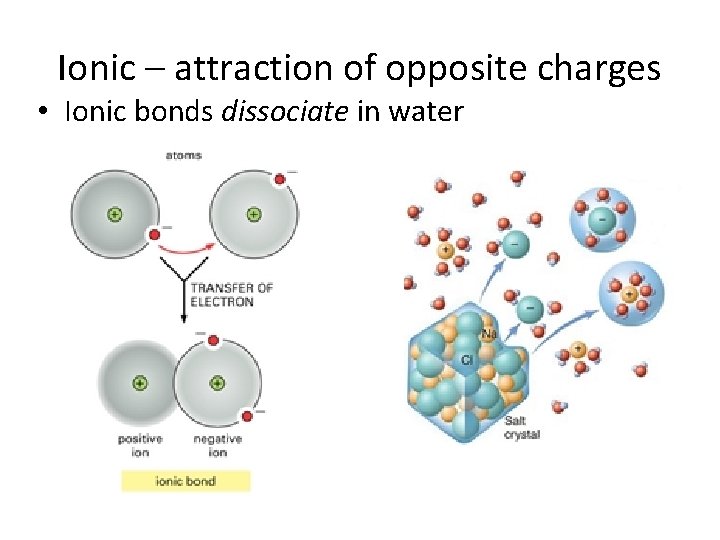
Ionic – attraction of opposite charges • Ionic bonds dissociate in water
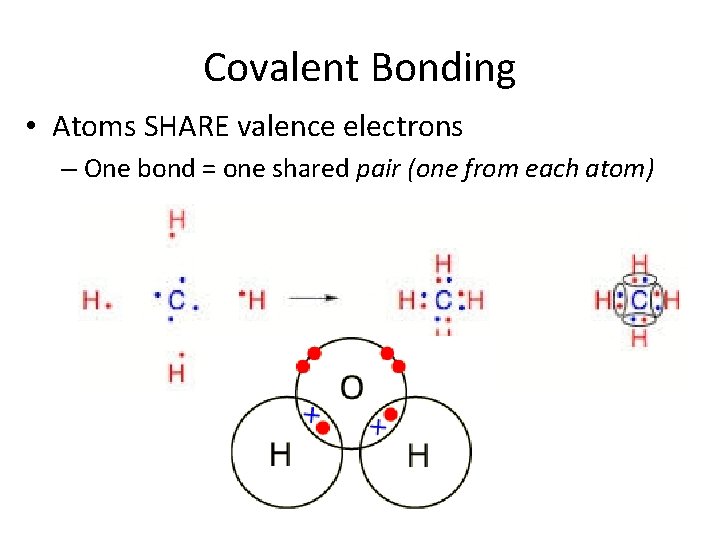
Covalent Bonding • Atoms SHARE valence electrons – One bond = one shared pair (one from each atom)
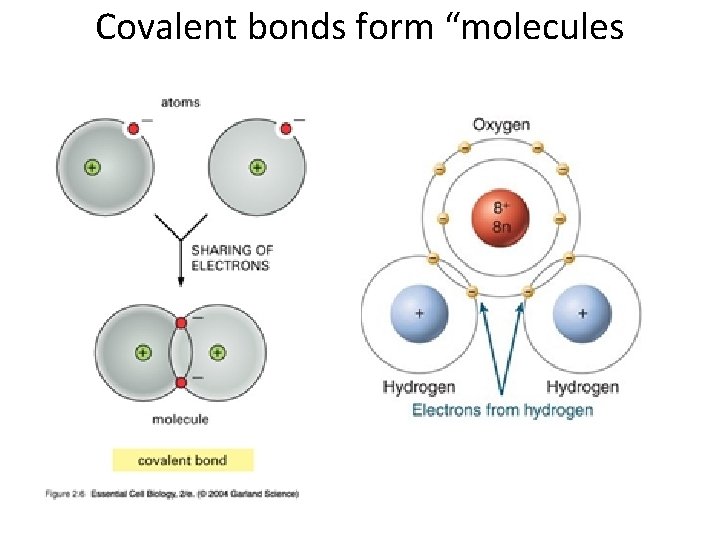
Covalent bonds form “molecules
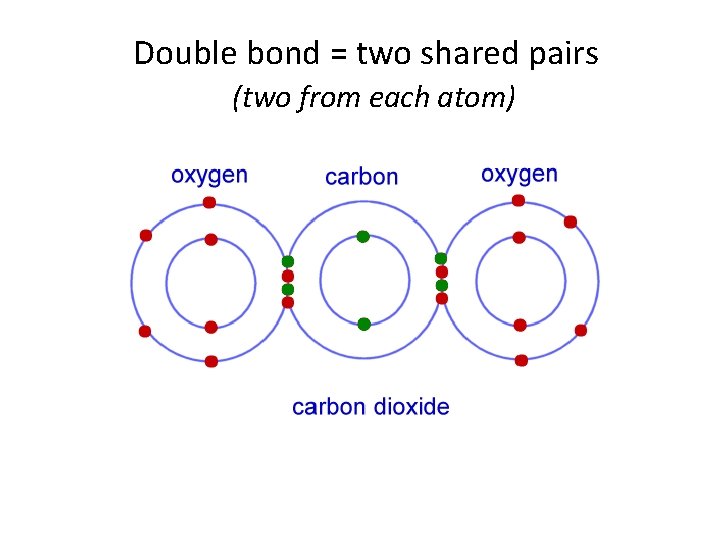
Double bond = two shared pairs (two from each atom)
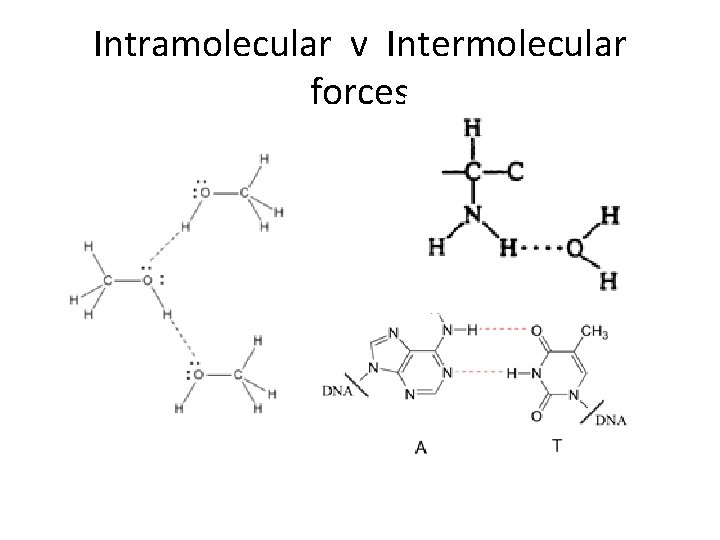
Intramolecular v Intermolecular forces
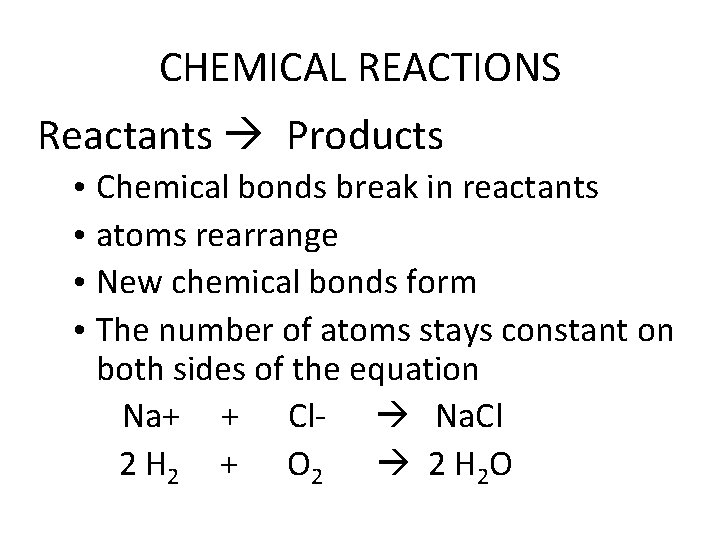
CHEMICAL REACTIONS Reactants Products • Chemical bonds break in reactants • atoms rearrange • New chemical bonds form • The number of atoms stays constant on both sides of the equation Na+ + Cl Na. Cl 2 H 2 + O 2 2 H 2 O
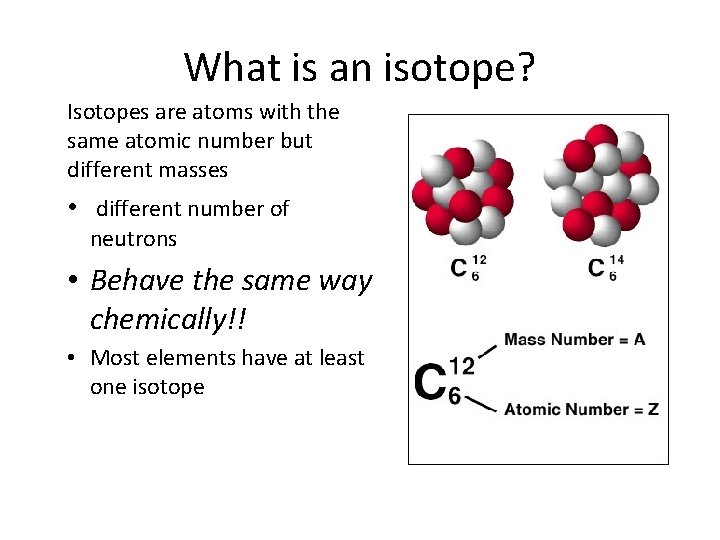
What is an isotope? Isotopes are atoms with the same atomic number but different masses • different number of neutrons • Behave the same way chemically!! • Most elements have at least one isotope
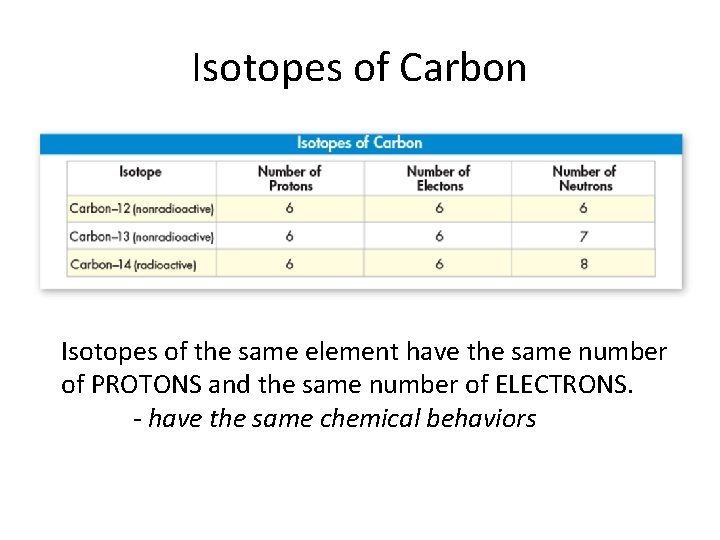
Isotopes of Carbon Isotopes of the same element have the same number of PROTONS and the same number of ELECTRONS. have the same chemical behaviors
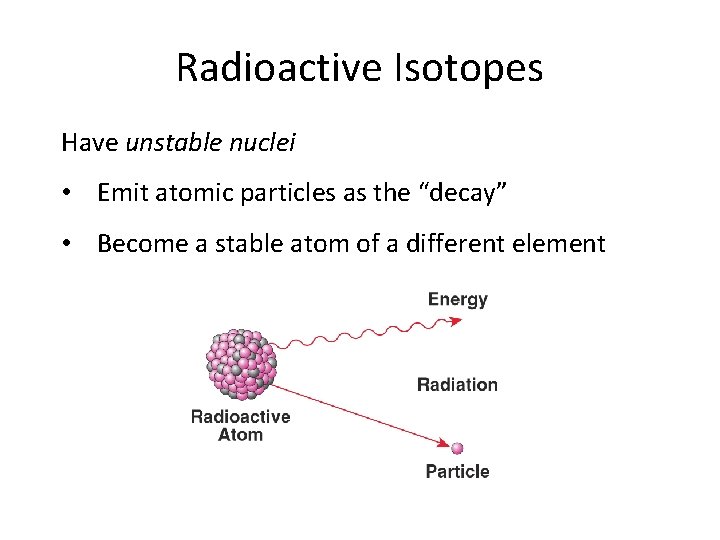
Radioactive Isotopes Have unstable nuclei • Emit atomic particles as the “decay” • Become a stable atom of a different element

Radioactive isotopes • Also emit radiation • at a steady rate, called “half life” – Half life: time for ½ of a sample to decay – Ex. Half life of C 14 is 5700 years • Can use radiation for many purposes

DATE FOSSILS Carbon 14 and other isotopes • measure amount of C 14 present in a fossil • compare to amount of present when it died • tells us how old the fossil is

Carbon Dating Fossils C 14 in atmosphere C 14 in carbon dioxide CO 2 used by plants in photosynthesis Some C 14 absorbed in ocean When animals die, no more C 14 taken in Some in animals after eating plants
Use as “tags” or “tracers” • Same chemical properties as stable isotopes • Used the same in life processes • Can show atoms and molecules are used in living things – Carbon 14 • showed how plants make food from CO 2 – Radioactive sulfur and phosphorus • showed how viruses enter cells
PET Scan MEDICAL DIAGNOSIS PET scan = Positron Emission Tomography • Radioisotope into blood collects in tissues • Amount of radiation shows organ function more active cells use more food and energy (ex. cancer cells) more active cells emit more radiation ”hot spot”
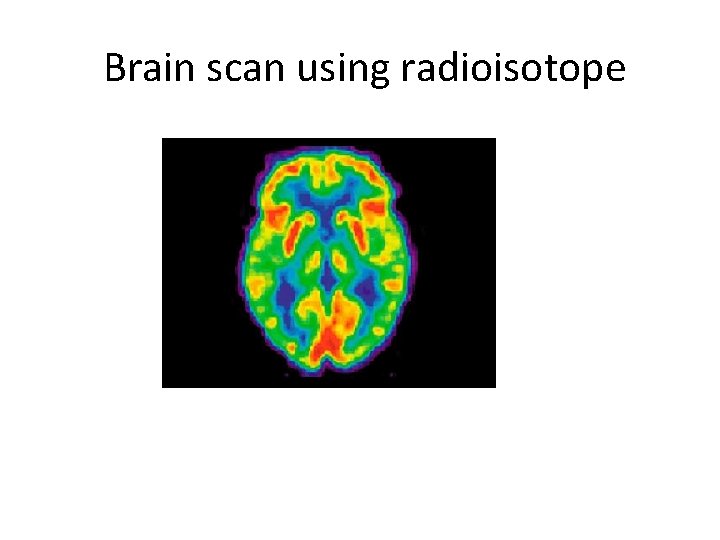
Brain scan using radioisotope
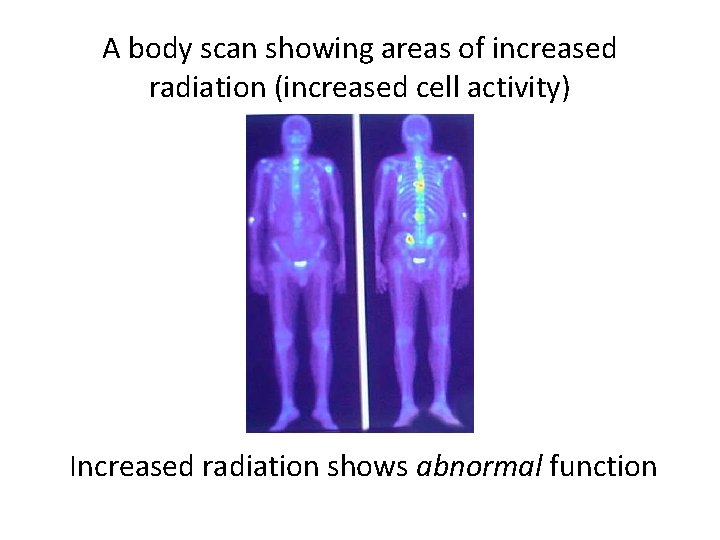
A body scan showing areas of increased radiation (increased cell activity) Increased radiation shows abnormal function
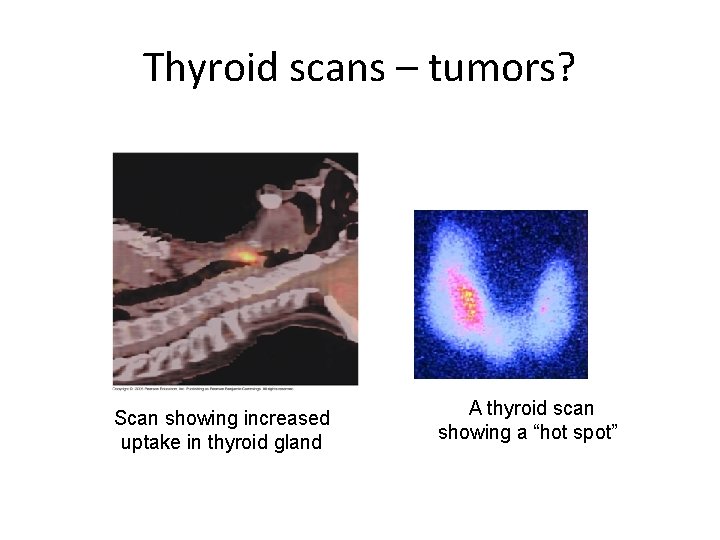
Thyroid scans – tumors? Scan showing increased uptake in thyroid gland A thyroid scan showing a “hot spot”
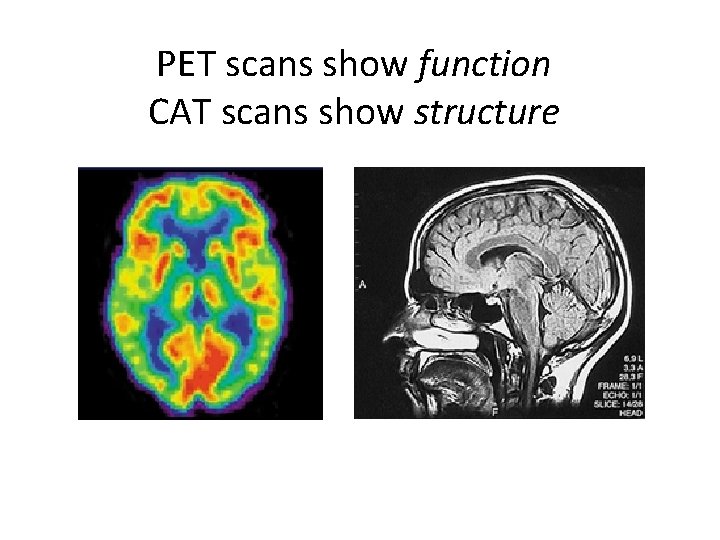
PET scans show function CAT scans show structure
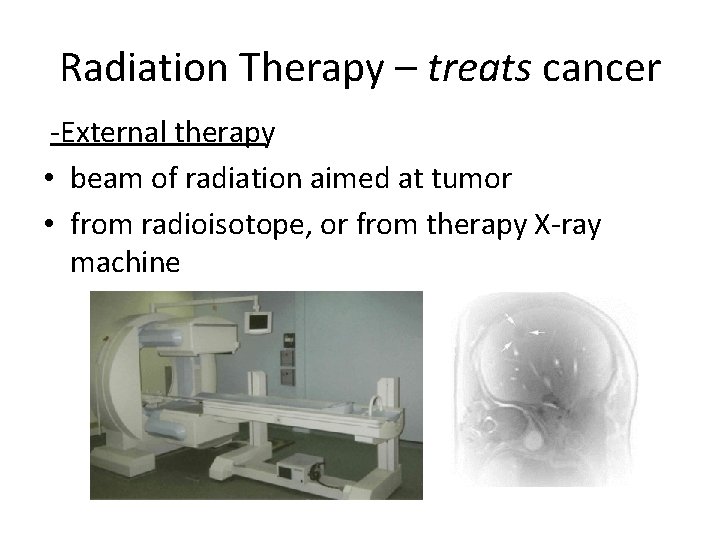
Radiation Therapy – treats cancer External therapy • beam of radiation aimed at tumor • from radioisotope, or from therapy X ray machine
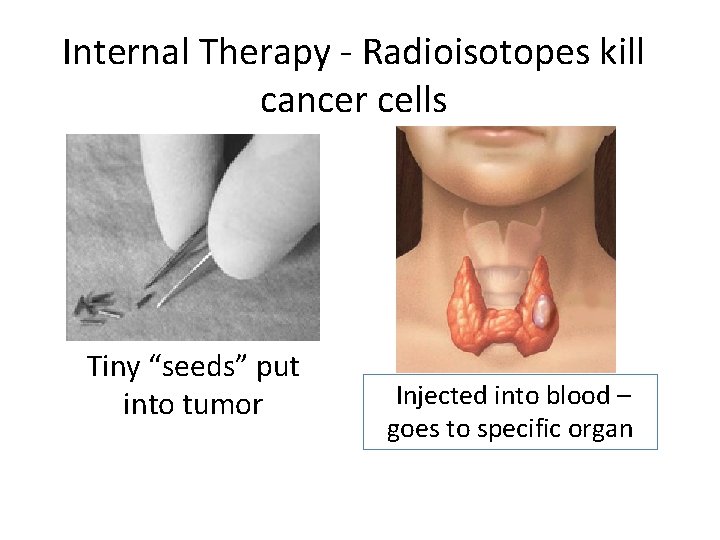
Internal Therapy Radioisotopes kill cancer cells Tiny “seeds” put into tumor Injected into blood – goes to specific organ
• • Uses in Industry Nuclear energy Sterilize surgery instruments Kill bacteria in food Long life batteries
- Slides: 31