COVID19 VACCINES AND THE ROLE OF THE PHARMACIST





















- Slides: 35

COVID-19 VACCINES AND THE ROLE OF THE PHARMACIST: Six Months on the Public Health Front-line Susan Conway, Pharm. D. , BCPS, FASHP Professor and Director of Experiential Education, University of Oklahoma College of Pharmacy Clinical Pharmacy Specialist, INTEGRIS Health Comprehensive Medication Management Service

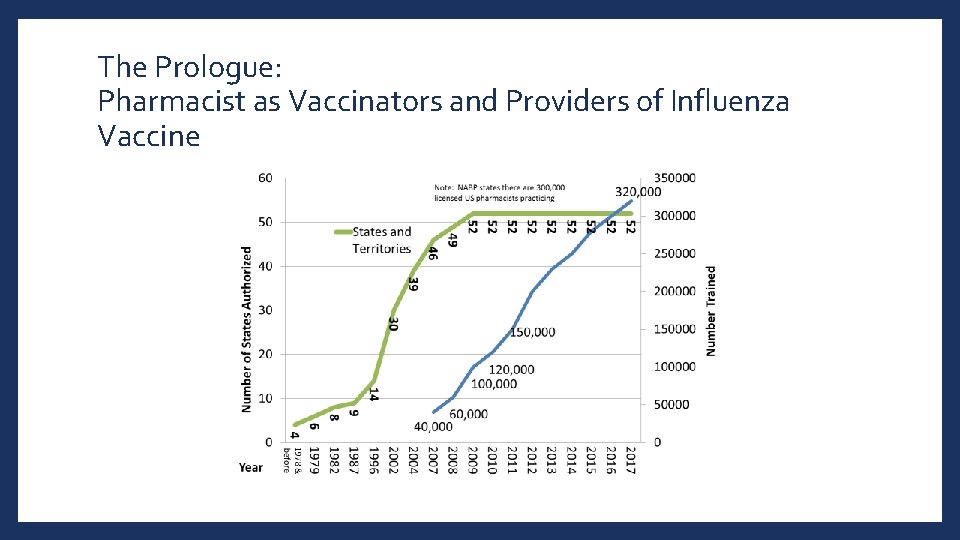
The Prologue: Pharmacist as Vaccinators and Providers of Influenza Vaccine

Oklahoma Pharmacists as Vaccinators • Rules and regulations passed in 2003 • D. Ph. shall administer immunizations on the order of a prescribing practitioner • Agreements will be allowed between an OK pharmacist and physician • Requirements for Immunizing D. Ph. • Complete approved training course • Register with OSBP for Immunization Permit and pay one-time fee of $25 • Maintain ongoing competence i. e. CPR & CE 3 https: //www. ok. gov/pharmacy/documents/2020%20 Rule%20 Book. pdf

Oklahoma Pharmacy Interns • May immunize if completed training course and working under Oklahoma D. Ph. with immunization permit • Exempt from registration https: //www. ok. gov/pharmacy/documents/2020%20 Rule%20 Book. pdf

Public Readiness and Emergency Preparedness Act (PREP Act) • In May 2020, the CDC reported drop in routine childhood immunizations • Amended PREP Act to increase access to childhood vaccines and decrease the risk of vaccine-preventable disease outbreaks • Authorized State-licensed pharmacists and interns to administer vaccines to individuals ages 3 -18 years according to the CDC’s ACIP immunization schedules. • The licensed pharmacist must inform families of the importance of a wellchild visit with a licensed PCP https: //www. hhs. gov/about/news/2020/08/19/hhs-expands-access-childhood-vaccines-during-covid-19 -pandemic. html

PREP Act Amendment: October 2020 • Authorizes pharmacy technicians acting under the supervision of a qualified pharmacist to administer to FDA-authorized COVID-19 vaccines to persons > 3 years and to administer FDA-licensed vaccines to persons ages 3 -18 according to ACIP’s standard immunization schedule • The vaccination must be ordered by the supervising qualified pharmacist. • The supervising qualified pharmacist must be readily and immediately available. • The technician must complete 2 hours ACPE training program including a handson injection technique and the recognition and treatment of emergency reactions to vaccines. https: //www. hhs. gov/sites/default/files/prep-act-guidance. pdf 6

7 PREP Act Amendment: October 2020 • The technician must have a current certificate in basic CPR • The supervising pharmacist must comply with recordkeeping and reporting requirements, including informing the patient’s PCP when available and submitting the required OSIIS (vaccine registry). • The supervising pharmacist is responsible for complying with requirements related to reporting adverse events. • The pharmacy technician must inform pediatric patients and their caregiver of the importance of a well-child visit with a pediatrician/PCP. https: //www. hhs. gov/sites/default/files/prep-act-guidance. pdf

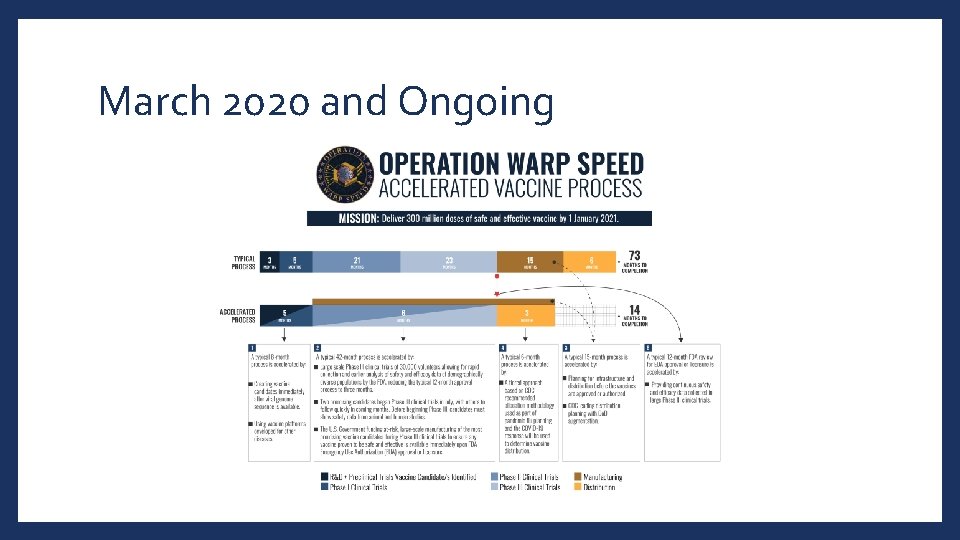
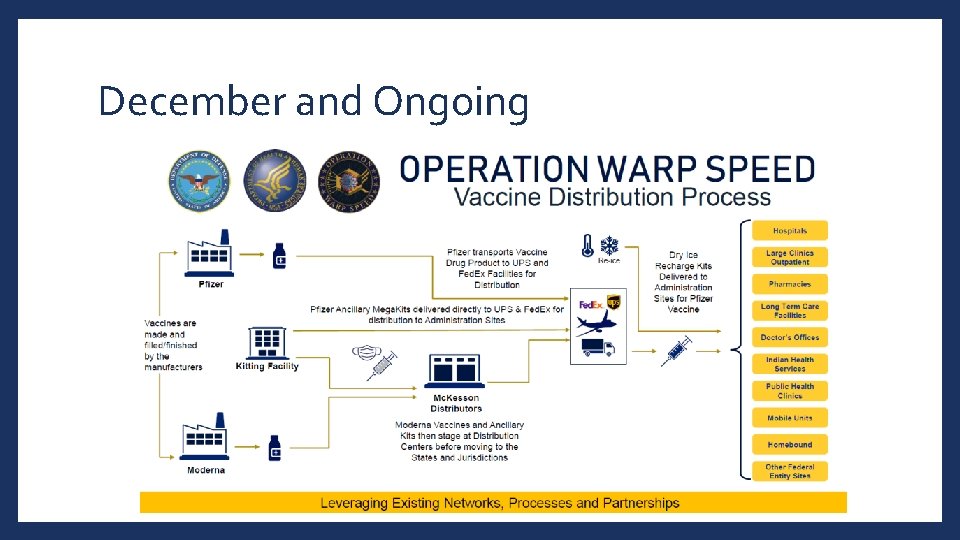
March 2020 and Ongoing

October: OUM and OUHSC Push Partner Committees § 17 members from administration, pharmacy, medicine, nursing, IT, risk management, communications, legal § Priority topics § § § § Recipient list Storage Location Documentation POD staffing and logistics Safety Communications

November and ongoing: OSHD and OCCHD meetings § Routine CDC and health department debriefings and directives § Operation Ludacris Speed tabletop exercise 11. 4. 21 Debriefing § Scenario workshopping: Limited supply, great demand § Scenario: workshopping: Limited demand, great supply § § Transitioned from very limited initial metro push partners to wheel and spoke model

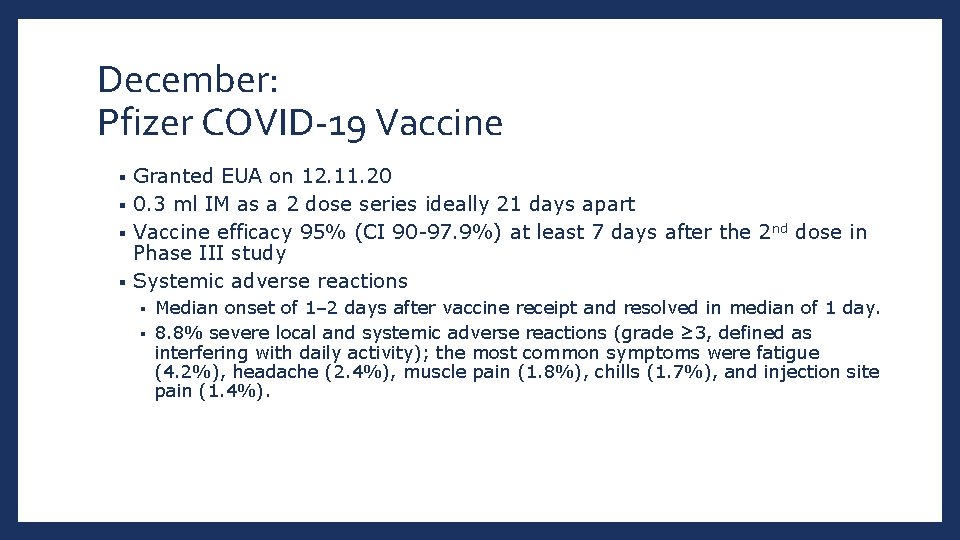
December: Pfizer COVID-19 Vaccine Granted EUA on 12. 11. 20 § 0. 3 ml IM as a 2 dose series ideally 21 days apart § Vaccine efficacy 95% (CI 90 -97. 9%) at least 7 days after the 2 nd dose in Phase III study § Systemic adverse reactions § Median onset of 1– 2 days after vaccine receipt and resolved in median of 1 day. § 8. 8% severe local and systemic adverse reactions (grade ≥ 3, defined as interfering with daily activity); the most common symptoms were fatigue (4. 2%), headache (2. 4%), muscle pain (1. 8%), chills (1. 7%), and injection site pain (1. 4%). §

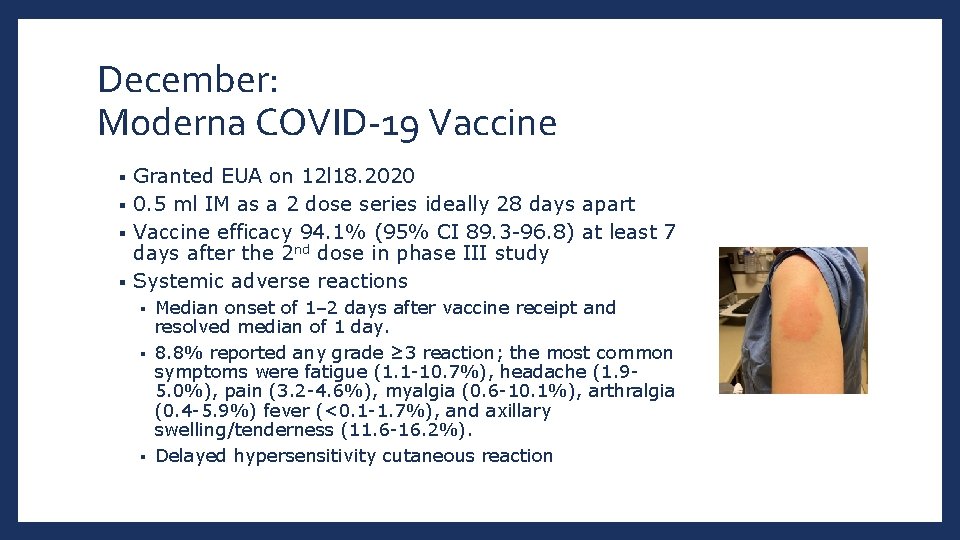
December: Moderna COVID-19 Vaccine Granted EUA on 12 l 18. 2020 § 0. 5 ml IM as a 2 dose series ideally 28 days apart § Vaccine efficacy 94. 1% (95% CI 89. 3 -96. 8) at least 7 days after the 2 nd dose in phase III study § Systemic adverse reactions § Median onset of 1– 2 days after vaccine receipt and resolved median of 1 day. § 8. 8% reported any grade ≥ 3 reaction; the most common symptoms were fatigue (1. 1 -10. 7%), headache (1. 95. 0%), pain (3. 2 -4. 6%), myalgia (0. 6 -10. 1%), arthralgia (0. 4 -5. 9%) fever (<0. 1 -1. 7%), and axillary swelling/tenderness (11. 6 -16. 2%). § Delayed hypersensitivity cutaneous reaction §

December and Ongoing

December and Ongoing: Safety Monitoring

December: Federal Vaccine Allocations • US Department of Veteran’s Affairs • Indian Health Service • National Pharmacy Program • • Activated week of 12/22 for LTC in Oklahoma Listed partners for Oklahoma • • Costco Wholesale Corp. CPESN USA, LLC CVS Pharmacy, Inc. Good Neighbor Pharmacy and Amerisource. Bergen Drug Corporation’s pharmacy services administrative organization (PSAO), Elevate Provider Health Mart Pharmacies Leader. NET nd The Medicine Shoppe Pharmacy, Cardinal Health’s SAOs Topco Associates, LLC Walgreens Walmart, Inc. (including Sam’s Club)

December 15

December: INTEGRIS Makes Headlines First dose in Oklahoma! The 6 th Pfizer dose!!

December: OU Health COVID-19 Vaccine PODs • Medical Director Approved-Protocol • Scheduling: Microsoft bookings • Location and hours: Social distancing, ED access, employee access • Vaccine storage and prep • Vaccine clinic staffing • Documentation: REDCap • OSIIS data entry team • Daily debriefing meeting

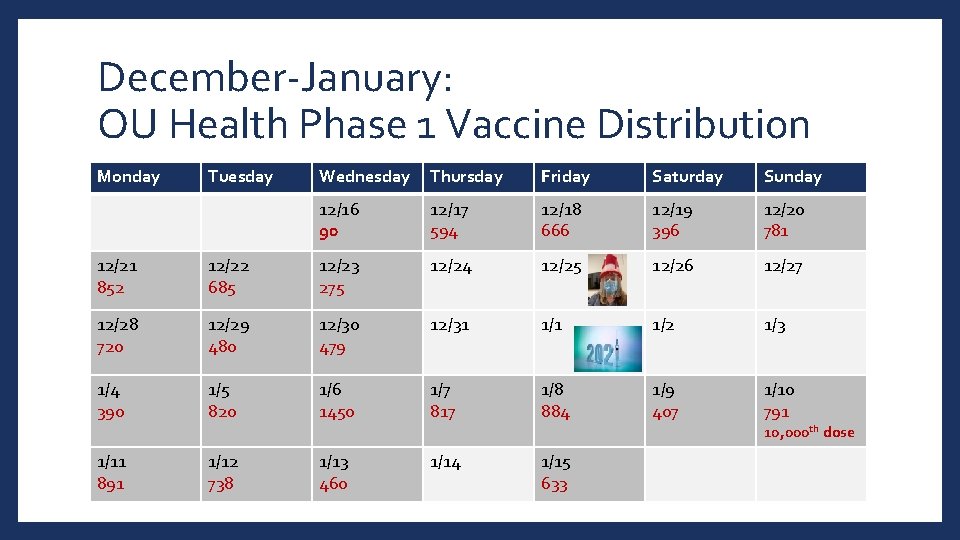
December-January: OU Health Phase 1 Vaccine Distribution Monday Tuesday Wednesday Thursday Friday Saturday Sunday 12/16 90 12/17 594 12/18 666 12/19 396 12/20 781 12/21 852 12/22 685 12/23 275 12/24 12/25 12/26 12/27 12/28 720 12/29 480 12/30 479 12/31 1/2 1/3 1/4 390 1/5 820 1/6 1450 1/7 817 1/8 884 1/9 407 1/10 791 1/11 891 1/12 738 1/13 460 1/14 1/15 633 10, 000 th dose

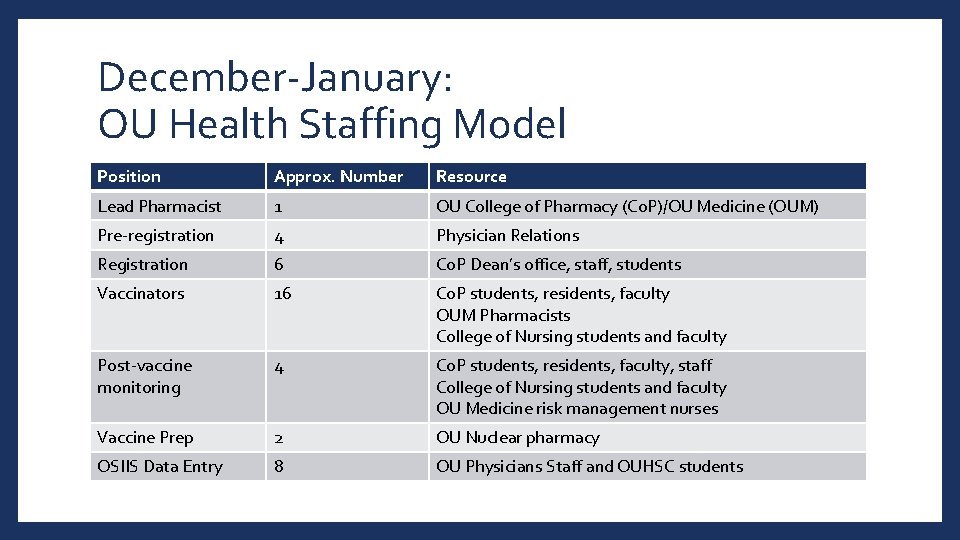
December-January: OU Health Staffing Model Position Approx. Number Resource Lead Pharmacist 1 OU College of Pharmacy (Co. P)/OU Medicine (OUM) Pre-registration 4 Physician Relations Registration 6 Co. P Dean’s office, staff, students Vaccinators 16 Co. P students, residents, faculty OUM Pharmacists College of Nursing students and faculty Post-vaccine monitoring 4 Co. P students, residents, faculty, staff College of Nursing students and faculty OU Medicine risk management nurses Vaccine Prep 2 OU Nuclear pharmacy OSIIS Data Entry 8 OU Physicians Staff and OUHSC students

December and Ongoing: Pharmacist Staffing Models across Oklahoma • Workers Pharmacies and health-systems • Pharmacists, interns, technicians, support staff • • Volunteers County health department • Oklahoma Medical Reserve Corps: https: //www. okmrc. org/ • • Roles Dose preparation • Vaccinator • POD leadership •

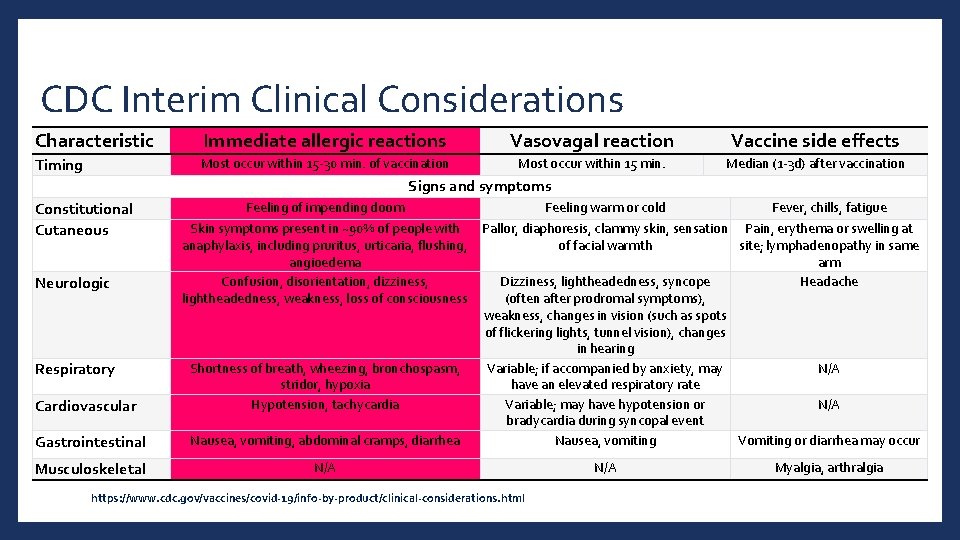
CDC Interim Clinical Considerations Characteristic Immediate allergic reactions Vasovagal reaction Vaccine side effects Timing Most occur within 15 -30 min. of vaccination Most occur within 15 min. Median (1 -3 d) after vaccination Signs and symptoms Constitutional Cutaneous Neurologic Respiratory Feeling of impending doom Skin symptoms present in ~90% of people with anaphylaxis, including pruritus, urticaria, flushing, angioedema Confusion, disorientation, dizziness, lightheadedness, weakness, loss of consciousness Cardiovascular Shortness of breath, wheezing, bronchospasm, stridor, hypoxia Hypotension, tachycardia Gastrointestinal Nausea, vomiting, abdominal cramps, diarrhea Musculoskeletal N/A Feeling warm or cold Fever, chills, fatigue Pallor, diaphoresis, clammy skin, sensation Pain, erythema or swelling at of facial warmth site; lymphadenopathy in same arm Dizziness, lightheadedness, syncope Headache (often after prodromal symptoms), weakness, changes in vision (such as spots of flickering lights, tunnel vision), changes in hearing Variable; if accompanied by anxiety, may N/A have an elevated respiratory rate Variable; may have hypotension or N/A bradycardia during syncopal event Nausea, vomiting Vomiting or diarrhea may occur https: //www. cdc. gov/vaccines/covid-19/info-by-product/clinical-considerations. html N/A Myalgia, arthralgia

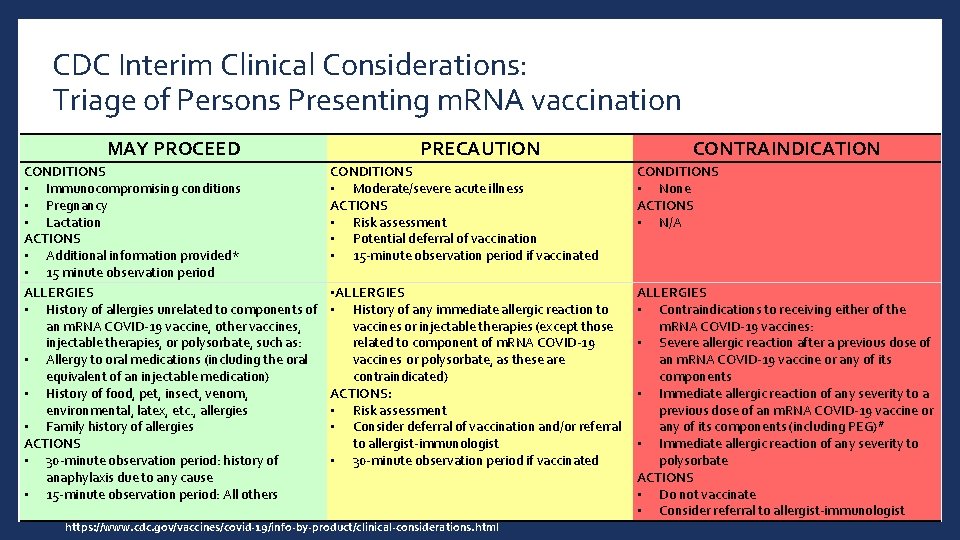
CDC Interim Clinical Considerations: Triage of Persons Presenting m. RNA vaccination MAY PROCEED CONDITIONS • Immunocompromising conditions • Pregnancy • Lactation ACTIONS • Additional information provided* • 15 minute observation period ALLERGIES • History of allergies unrelated to components of an m. RNA COVID-19 vaccine, other vaccines, injectable therapies, or polysorbate, such as: • Allergy to oral medications (including the oral equivalent of an injectable medication) • History of food, pet, insect, venom, environmental, latex, etc. , allergies • Family history of allergies ACTIONS • 30 -minute observation period: history of anaphylaxis due to any cause • 15 -minute observation period: All others PRECAUTION CONTRAINDICATION CONDITIONS • Moderate/severe acute illness ACTIONS • Risk assessment • Potential deferral of vaccination • 15 -minute observation period if vaccinated CONDITIONS • None ACTIONS • N/A • ALLERGIES • History of any immediate allergic reaction to vaccines or injectable therapies (except those related to component of m. RNA COVID-19 vaccines or polysorbate, as these are contraindicated) ACTIONS: • Risk assessment • Consider deferral of vaccination and/or referral to allergist-immunologist • 30 -minute observation period if vaccinated ALLERGIES • Contraindications to receiving either of the m. RNA COVID-19 vaccines: • Severe allergic reaction after a previous dose of an m. RNA COVID-19 vaccine or any of its components • Immediate allergic reaction of any severity to a previous dose of an m. RNA COVID-19 vaccine or any of its components (including PEG)# • Immediate allergic reaction of any severity to polysorbate ACTIONS • Do not vaccinate • Consider referral to allergist-immunologist https: //www. cdc. gov/vaccines/covid-19/info-by-product/clinical-considerations. html

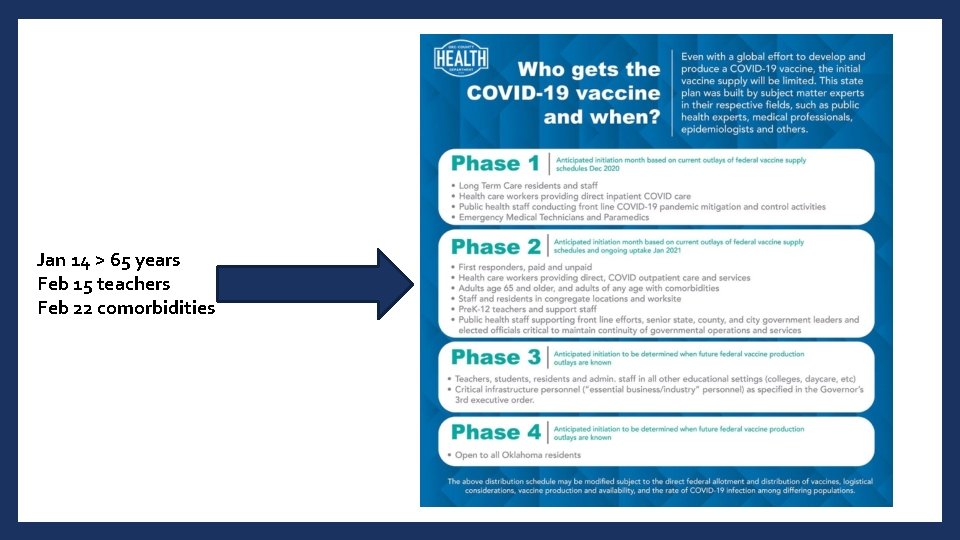
Jan 14 > 65 years Feb 15 teachers Feb 22 comorbidities

January and Ongoing: OU Health Expanding Staffing Model • Pharmacy-led clinics • Population OU Health/OUHSC employees & students • Community outreach strike team • OU Physicians-led clinics • • • Expanded staffing strategies • January APPE • IPPE • Added trainings for P 1 s, pharmacists, and pharmacy technicians • Class of 2025 Efficiencies in IT • • Patient/public clinics Staffing model • • • Population Nursing quality leadership IPE student leadership team OUM/SCC Pharmacist - vaccine prep Medical and nursing studentsvaccinators Students and employees-nonclinical roles Efficiencies in IT and adopting megapod workflows

January and Ongoing: Expanding Technology

February to April: OU Health Community Outreach Clinics • Reduced to weekly cadence • Local schools and churches • Outreach to underrepresented ethnicities • Addressing vaccine hesitancy and system barriers • Community volunteers for scheduling outreach and non-clinical vaccine POD roles

February and Ongoing: Community Pharmacies Offering Vaccine

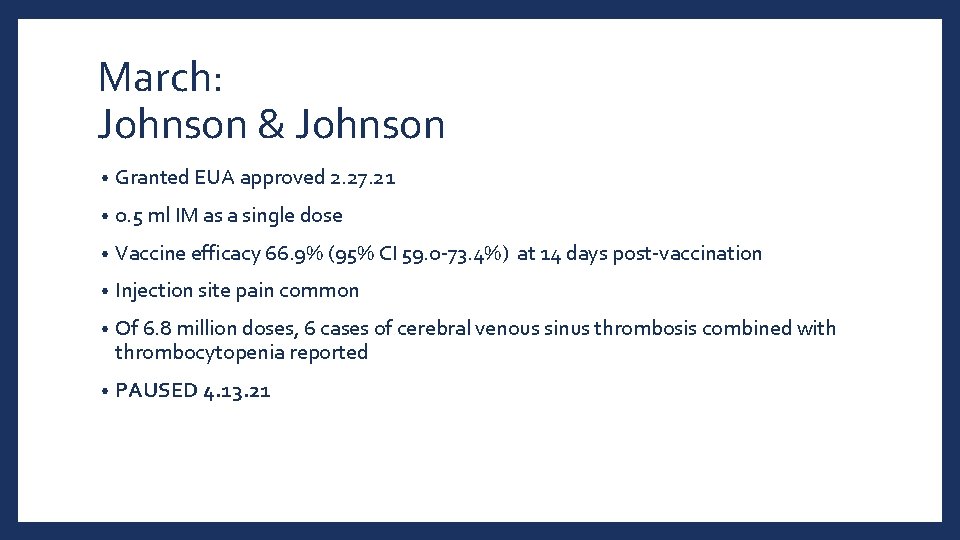
March: Johnson & Johnson • Granted EUA approved 2. 27. 21 • 0. 5 ml IM as a single dose • Vaccine efficacy 66. 9% (95% CI 59. 0 -73. 4%) at 14 days post-vaccination • Injection site pain common • Of 6. 8 million doses, 6 cases of cerebral venous sinus thrombosis combined with thrombocytopenia reported • PAUSED 4. 13. 21

March to April: CDC Recommendations for Vaccinated People

March 10 th March 29

MARCHing On : More Mega Pods and Addressing Hesitancy

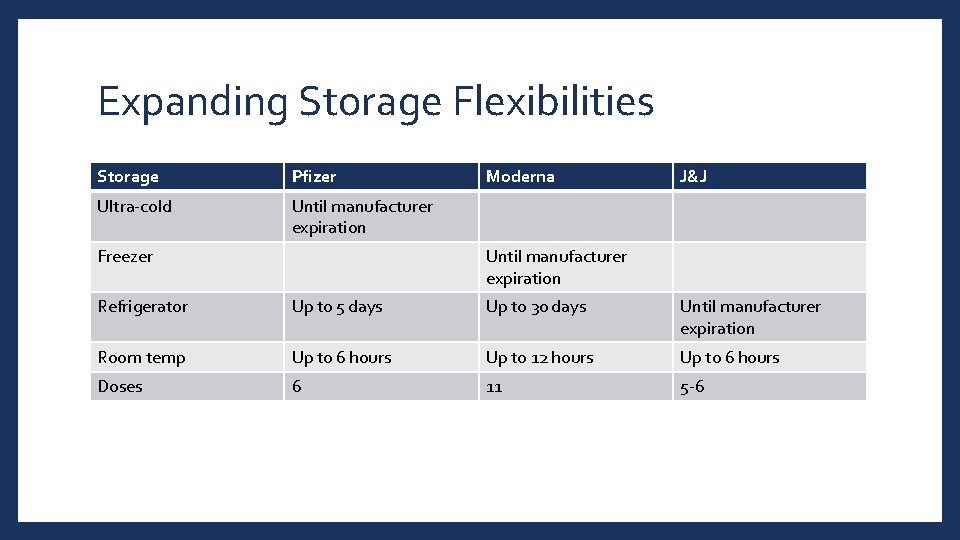
Expanding Storage Flexibilities Storage Pfizer Ultra-cold Until manufacturer expiration Freezer Moderna J&J Until manufacturer expiration Refrigerator Up to 5 days Up to 30 days Until manufacturer expiration Room temp Up to 6 hours Up to 12 hours Up to 6 hours Doses 6 11 5 -6

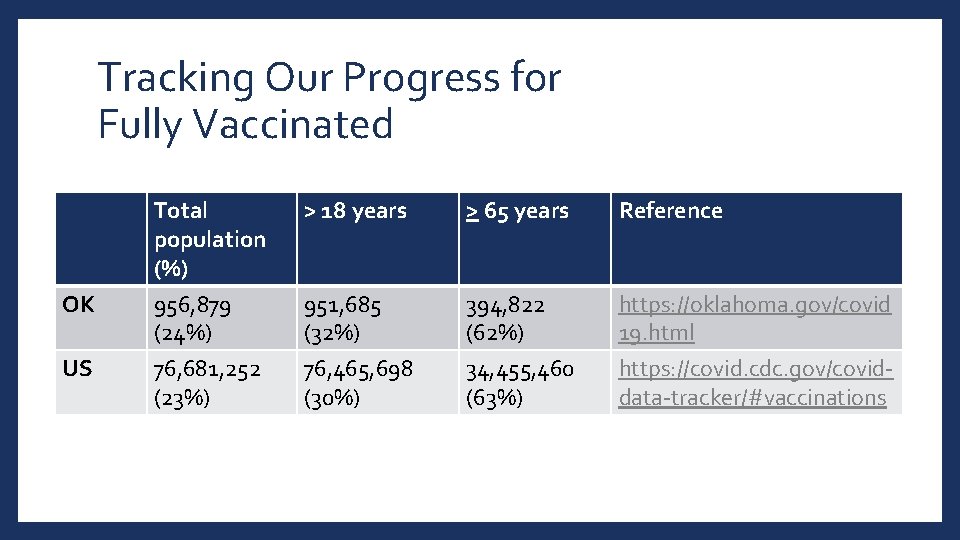
Tracking Our Progress for Fully Vaccinated OK US Total population (%) 956, 879 (24%) > 18 years > 65 years Reference 951, 685 (32%) 394, 822 (62%) https: //oklahoma. gov/covid 19. html 76, 681, 252 (23%) 76, 465, 698 (30%) 34, 455, 460 (63%) https: //covid. cdc. gov/coviddata-tracker/#vaccinations

Questions and Shares