Covalent Structures EQ How are the chemical formulas









![• Resonance structures: o Bracket the structure [ ] o Indicates resonant bonds • Resonance structures: o Bracket the structure [ ] o Indicates resonant bonds](https://slidetodoc.com/presentation_image_h/0c164c374957143add9c1597be53fcde/image-25.jpg)



- Slides: 28

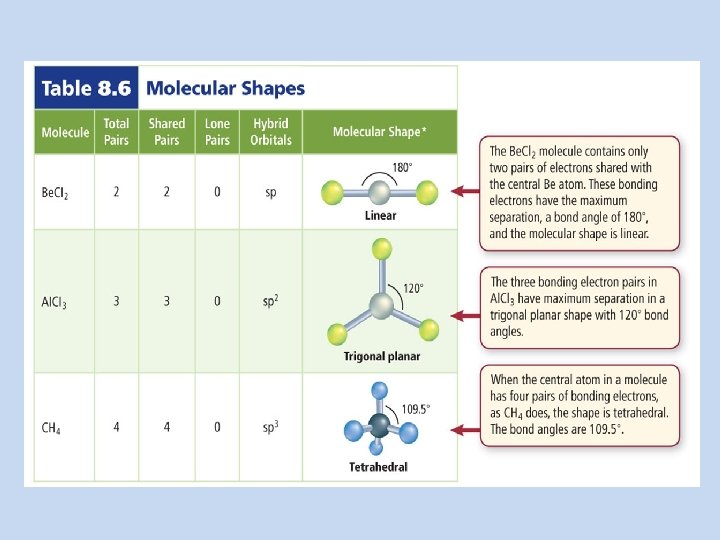
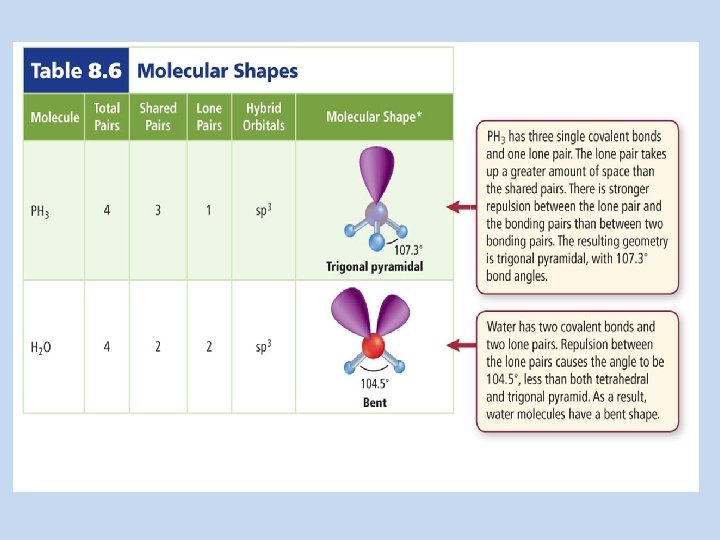
Covalent Structures EQ: How are the chemical formulas and chemical names written for covalent molecules? How do you draw VSEPR diagrams for covalent compounds? What are the names and bonding/lone pairs for each molecular shape?

Chemical Bonding • Lasting attraction between atoms, molecules or ions that results in formation of chemical compounds – Results from attraction of oppositely charged ions – Results from sharing of electrons

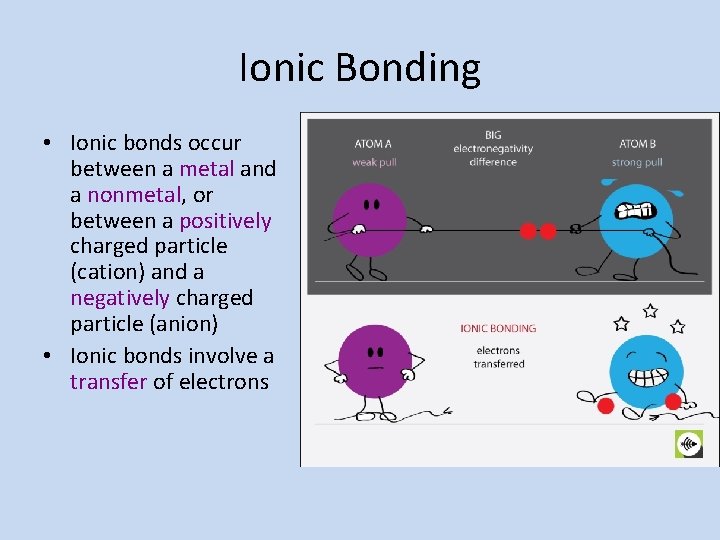
Ionic Bonding • Ionic bonds occur between a metal and a nonmetal, or between a positively charged particle (cation) and a negatively charged particle (anion) • Ionic bonds involve a transfer of electrons

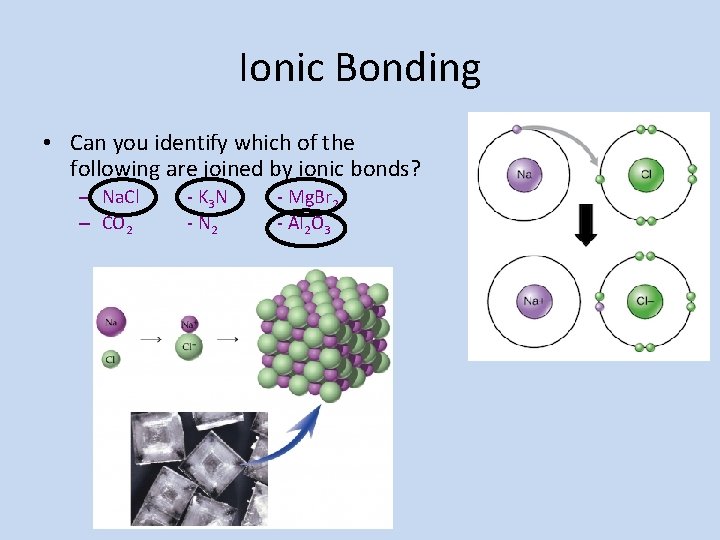
Ionic Bonding • Can you identify which of the following are joined by ionic bonds? – Na. Cl – CO 2 - K 3 N - N 2 - Mg. Br 2 - Al 2 O 3

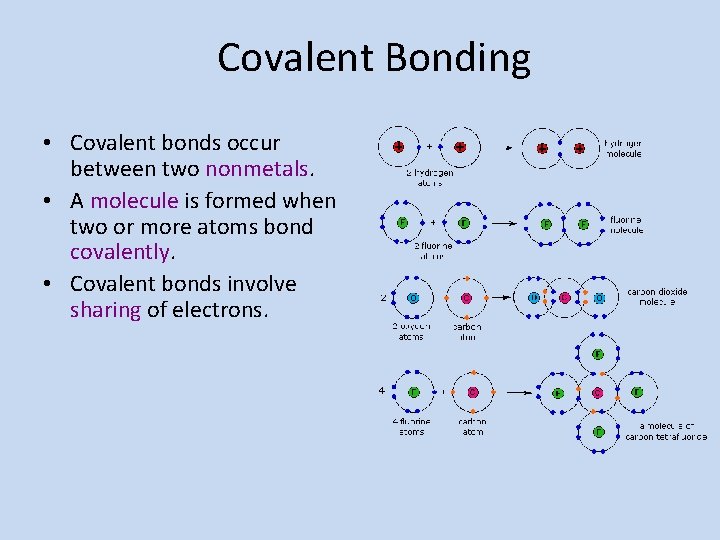
Covalent Bonding • Covalent bonds occur between two nonmetals. • A molecule is formed when two or more atoms bond covalently. • Covalent bonds involve sharing of electrons.

Ionic or Covalent? ? ? Which of the following are held together by ionic bonds and which are covalently bonded? A. Mg. SO 4 Ionic B. Dinitrogen tetroxide Covalent C. Aluminum chloride Ionic D. SO 2 Covalent E. Two fluorine atoms Covalent

Diatomic Molecules • Certain elements occur in nature as diatomic molecules and not as single atoms (more stable as the molecule) • Examples: – H 2, N 2, O 2, – F 2, Cl 2, Br 2, I 2 • Diatomic Molecules are bonded together by covalent bonds.

Covalent Names • Common Names: – Ozone - O 3 – Methane - CH 4 – Hydrogen peroxide - H 2 O 2 – Water - H 2 O – Ammonia - NH 3

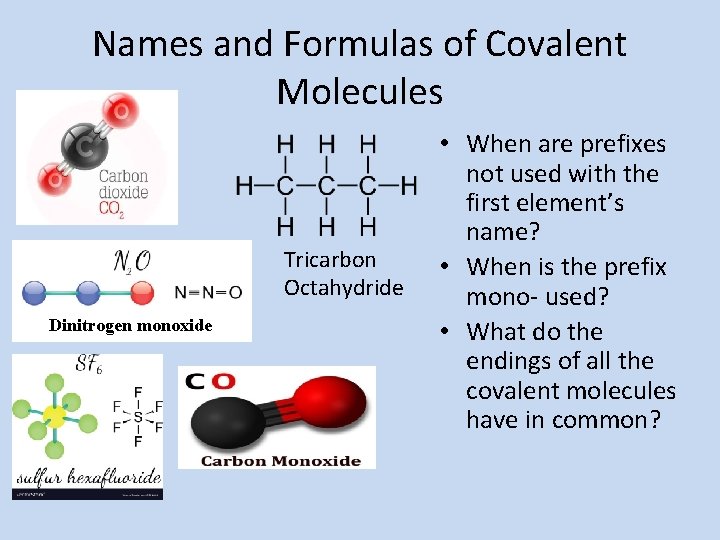
Names and Formulas of Covalent Molecules Tricarbon Octahydride Dinitrogen monoxide • When are prefixes not used with the first element’s name? • When is the prefix mono- used? • What do the endings of all the covalent molecules have in common?

Apply that Info!!! Identify if the name is written correctly for each of the following molecules. Correct if wrong. Disilicon A. Si 2 Br 6 – Silicon hexabromide B. NF 3 – Nitrogen trifluoride Correct! C. S 6 O – Hexasulfur oxide Hexasulfur monoxide D. P 4 S 5 – Tetraphosphide pentasulfide Tetraphosphorus E. B 6 Si – Hexaboron monosilicide pentasulfide Correct!!!

Name these molecules! What are the names of the following molecules? Nitrogen A. NO monoxide B. Xe. F 6 Xenon hexafluoride Phosphorus C. PCl 5 pentachloride

What are the rules? ? ? Discuss with your neighbor the rules on naming covalent molecules. • Elements written in same order as in formula • Prefixes indicate number of atoms • No “mono-” prefix on the 1 st element; prefixes always on 2 nd element • Ending of molecule is “-ide” • Drop the vowel on prefix (except “di

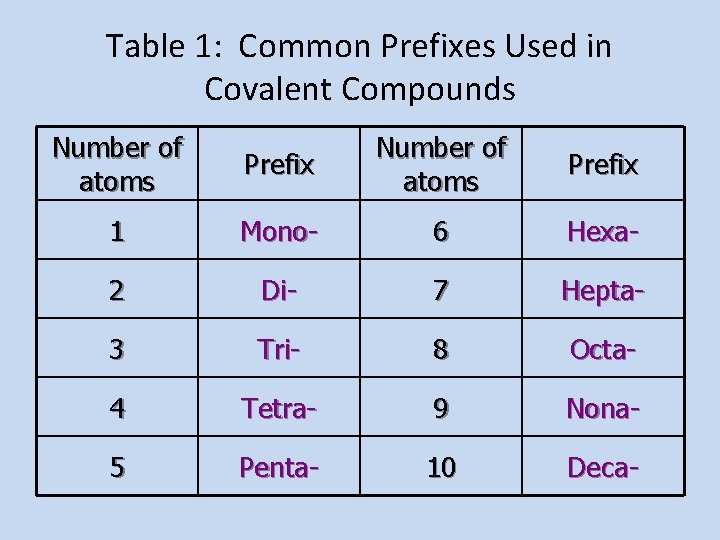
Table 1: Common Prefixes Used in Covalent Compounds Number of atoms Prefix 1 Mono- 6 Hexa- 2 Di- 7 Hepta- 3 Tri- 8 Octa- 4 Tetra- 9 Nona- 5 Penta- 10 Deca-

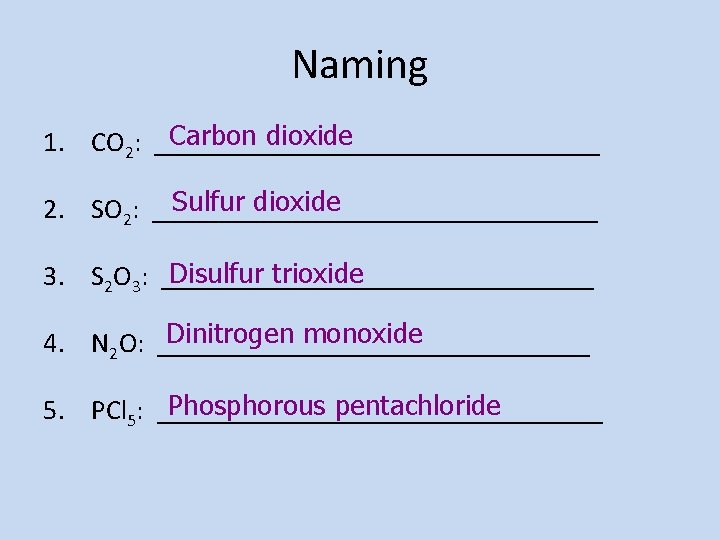
Naming Carbon dioxide 1. CO 2: ________________ Sulfur dioxide 2. SO 2: ________________ Disulfur trioxide 3. S 2 O 3: ________________ Dinitrogen monoxide 4. N 2 O: ________________ Phosphorous pentachloride 5. PCl 5: ________________

Formula Writing 1. Selenium hexafluoride: ______ Se. F 6 2. Si 2 Br 6 Disilicon hexabromide: _____ 3. B 2 Si Diboron monosilicide: _____ 4. IF 5 Iodine pentafluoride: ____ 5. B 6 Si Hexaboron monosilicide: ____

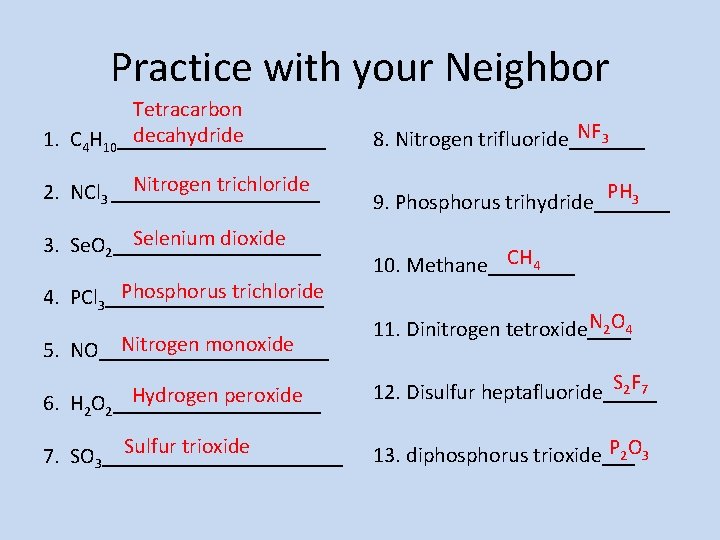
Practice with your Neighbor Tetracarbon decahydride 1. C 4 H 10__________ Nitrogen trichloride 2. NCl 3 __________ Selenium dioxide 3. Se. O 2__________ Phosphorus trichloride 4. PCl 3__________ Nitrogen monoxide 5. NO___________ Hydrogen peroxide 6. H 2 O 2__________ Sulfur trioxide 7. SO 3___________ NF 3 8. Nitrogen trifluoride_______ PH 3 9. Phosphorus trihydride_______ CH 4 10. Methane____ N 2 O 4 11. Dinitrogen tetroxide____ S 2 F 7 12. Disulfur heptafluoride_____ P 2 O 3 13. diphosphorus trioxide___

STOP!!! • Homework is page 5!!!

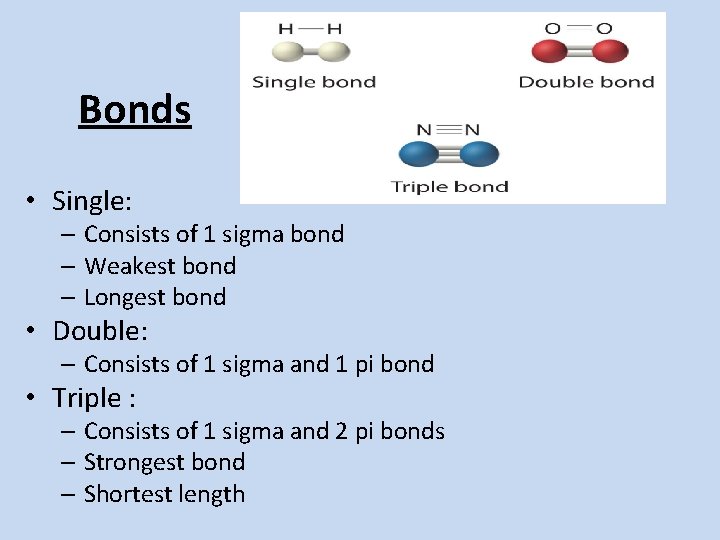
Bonds • Single: – Consists of 1 sigma bond – Weakest bond – Longest bond • Double: – Consists of 1 sigma and 1 pi bond • Triple : – Consists of 1 sigma and 2 pi bonds – Strongest bond – Shortest length



Electronegativity and Bond Polarity • What does electronegativity mean? – Ability to attract electrons Electron

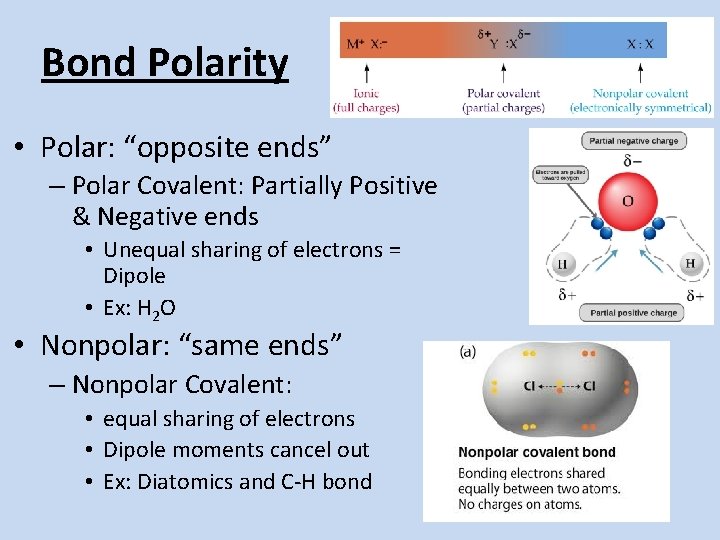
Bond Polarity • Polar: “opposite ends” – Polar Covalent: Partially Positive & Negative ends • Unequal sharing of electrons = Dipole • Ex: H 2 O • Nonpolar: “same ends” – Nonpolar Covalent: • equal sharing of electrons • Dipole moments cancel out • Ex: Diatomics and C-H bond

Dipoles • Slightly positive and slightly negative ends – Result from electrons being shared unequally in a polar covalent bond – More electronegative element gets – Less electronegative element gets

Molecular Polarity • Polar Molecule (H 2 O, CH 2 F 2, NH 3) – Different ends of the molecule OR – Lone pairs on central atom • Nonpolar Molecule (CO 2, CH 4) – Same ends on the molecule AND – No lone pairs on central atom
![Resonance structures o Bracket the structure o Indicates resonant bonds • Resonance structures: o Bracket the structure [ ] o Indicates resonant bonds](https://slidetodoc.com/presentation_image_h/0c164c374957143add9c1597be53fcde/image-25.jpg)
• Resonance structures: o Bracket the structure [ ] o Indicates resonant bonds with dotted line • Polyatomic Ions: o Bracket the structure [ ] o Indicate charge top right exterior of bracket

Practice Problems: Draw and Identify • O 3 • CO 32 • Si. S 2 • OF 2

Practice Problems: Draw and Identify • PCl 3 • NH 4+ • CH 3 Cl • SCl 2

STOP!!! • Homework – pages 7 -8