Covalent Nomenclature Naming Molecular Compounds Review p How





- Slides: 8

Covalent Nomenclature Naming Molecular Compounds

Review p How do we identify ionic formulas? n p How do we identify covalent formulas? n p Ionic formulas start with metals (or NH 4+1) Covalent formulas contain only non-metals or metalloids. How do we name ionic compounds? n n Identify the compound as binary or ternary. Identify the metal as fixed charge or variable charge. If the compound is binary, ends in –ide. If the compound is ternary, name the anion appropriately.

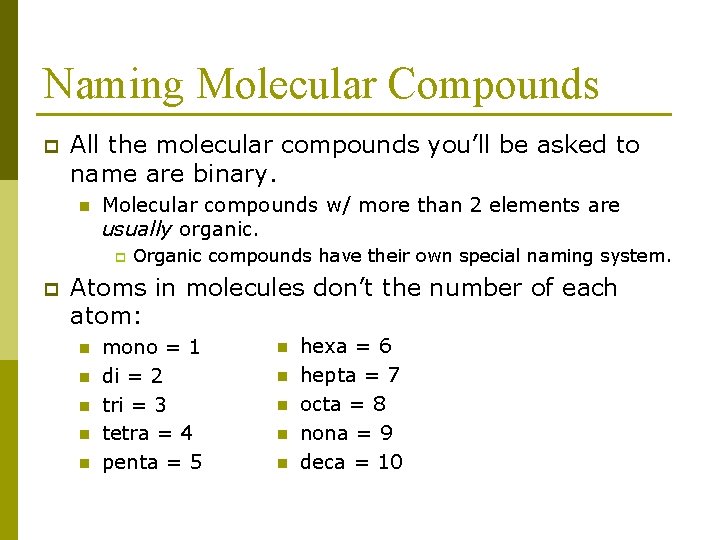
Naming Molecular Compounds p All the molecular compounds you’ll be asked to name are binary. n Molecular compounds w/ more than 2 elements are usually organic. p p Organic compounds have their own special naming system. Atoms in molecules don’t the number of each atom: n n n mono = 1 di = 2 tri = 3 tetra = 4 penta = 5 n n n hexa = 6 hepta = 7 octa = 8 nona = 9 deca = 10

Naming Molecular Compounds p Prefix for first element in compound. n Omit if it’s “mono”. Name first element. p Prefix for second element in compound. p Give stem of second element followed by suffix –ide. p n Examples: CO = carbon monoxide p CO 2 = carbon dioxide p N 2 O = dinitrogen monoxide p

Details and Exceptions p If the last letter of the prefix and the first letter of the element are both vowels, drop the last letter of the prefix. n n p CO = carbon monoxide, not carbon monooxide N 2 O 5 = dinitrogen pentoxide, not dinitrogen pentaoxide Some compounds are known only by their common names n n n H 2 O = water NH 3 = ammonia CH 4 = methane

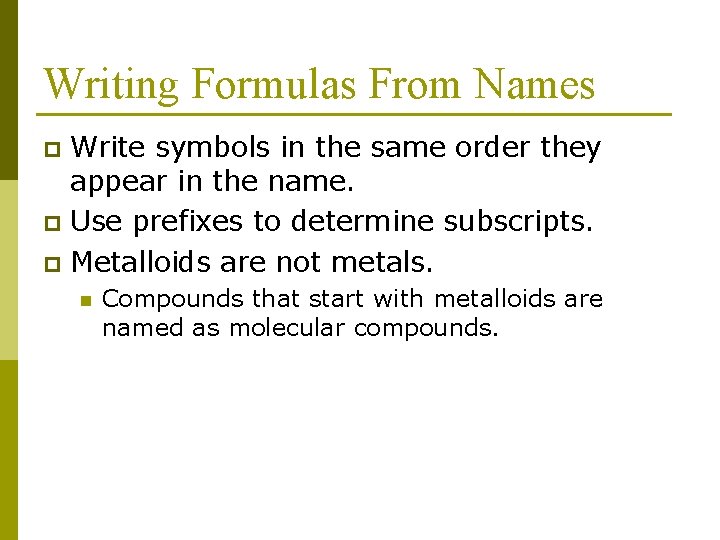
Writing Formulas From Names Write symbols in the same order they appear in the name. p Use prefixes to determine subscripts. p Metalloids are not metals. p n Compounds that start with metalloids are named as molecular compounds.

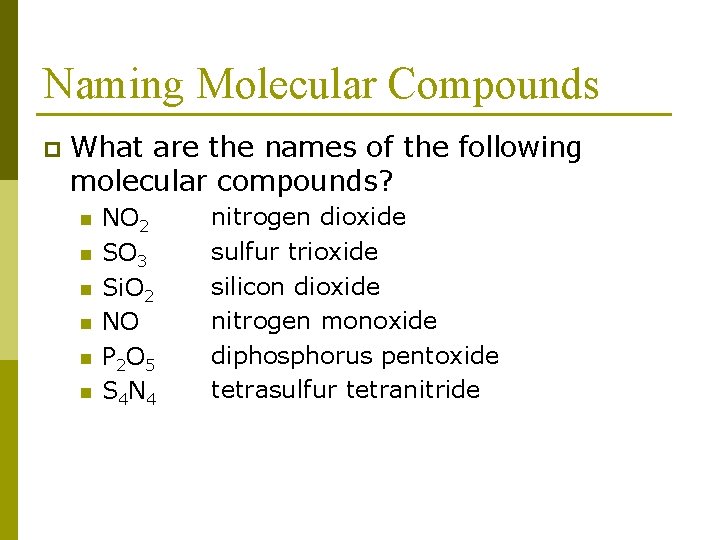
Naming Molecular Compounds p What are the names of the following molecular compounds? n n n NO 2 SO 3 Si. O 2 NO P 2 O 5 S 4 N 4 nitrogen dioxide sulfur trioxide silicon dioxide nitrogen monoxide diphosphorus pentoxide tetrasulfur tetranitride

Writing Formulas From Names p What are the formulas of the following molecular compounds? n n n n carbon tetrachloride phosphorus trichloride selenium dibromide dinitrogen tetroxide sulfur dioxide xenon tetrafluoride diantimony trioxide CCl 4 PCl 3 Se. Br 2 N 2 O 4 SO 2 Xe. F 4 Sb 2 O 3