Covalent Metallic Bonding Key Concept Covalent bonds form
Covalent & Metallic Bonding Key Concept: Covalent bonds form when atoms _____ share electrons. low Substances formed from covalent bonds tend to have ___ melting and boiling points and are ______ brittle in the solid state. liquids or ØMost covalent compounds are _____ gases at room temperature.
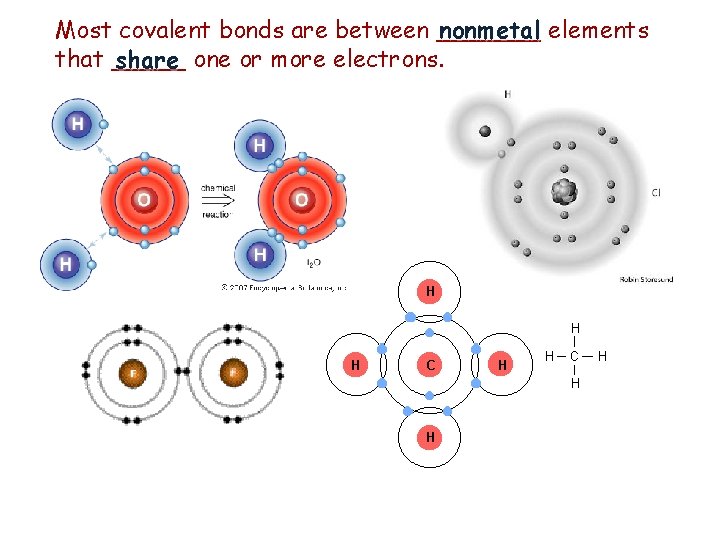
Most covalent bonds are between _______ nonmetal elements that _____ share one or more electrons.
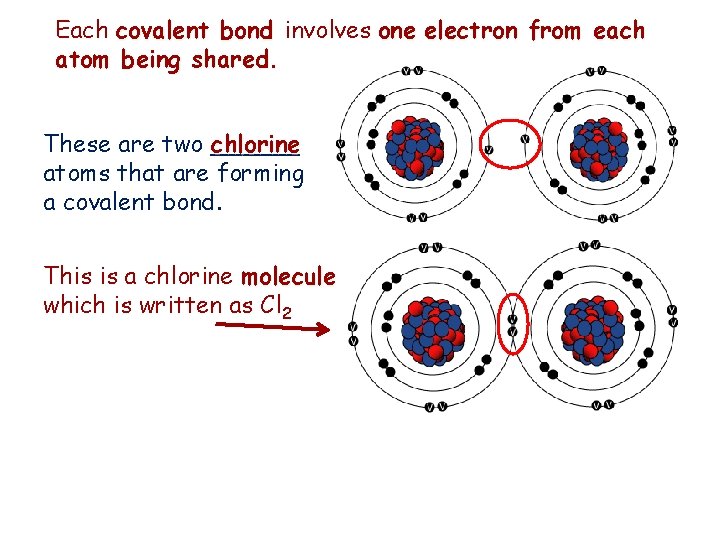
Each covalent bond involves one electron from each atom being shared. These are two ______ chlorine atoms that are forming a covalent bond. This is a chlorine molecule which is written as Cl 2
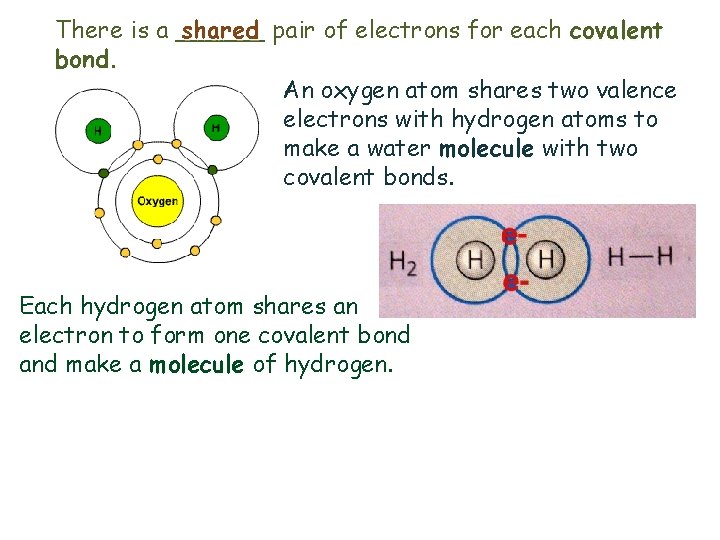
There is a ______ shared pair of electrons for each covalent bond. An oxygen atom shares two valence electrons with hydrogen atoms to make a water molecule with two covalent bonds. Each hydrogen atom shares an electron to form one covalent bond and make a molecule of hydrogen.
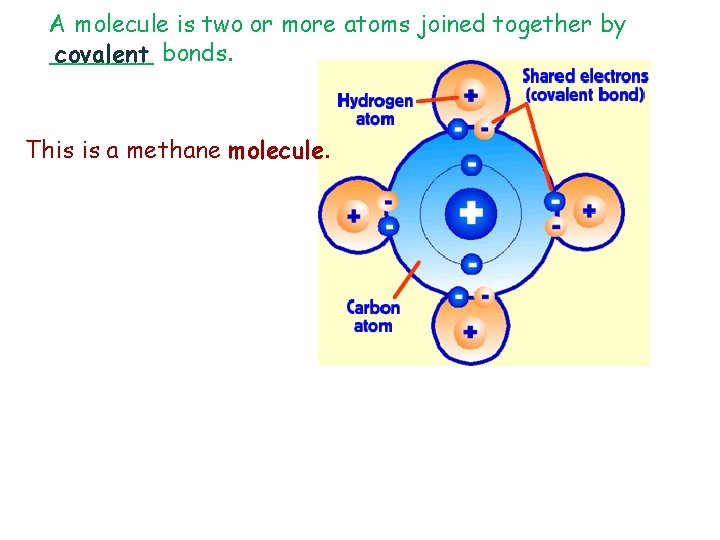
A molecule is two or more atoms joined together by _______ covalent bonds. This is a methane molecule
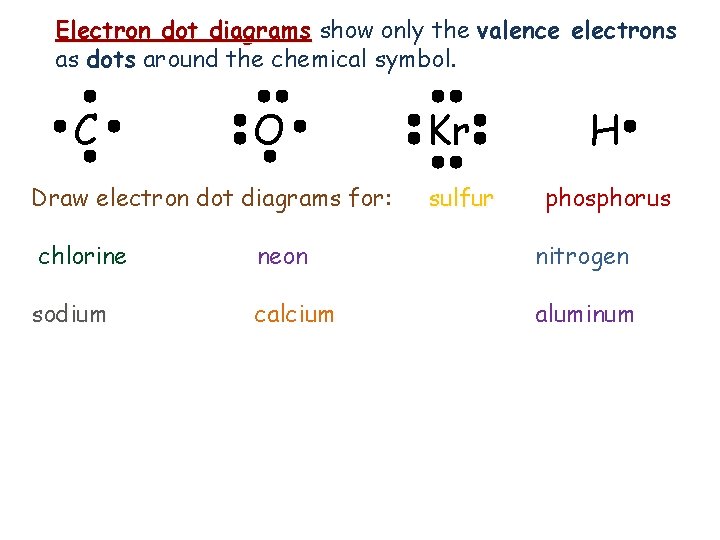
Electron dot diagrams show only the valence electrons as dots around the chemical symbol. C O Draw electron dot diagrams for: Kr sulfur H phosphorus chlorine neon nitrogen sodium calcium aluminum
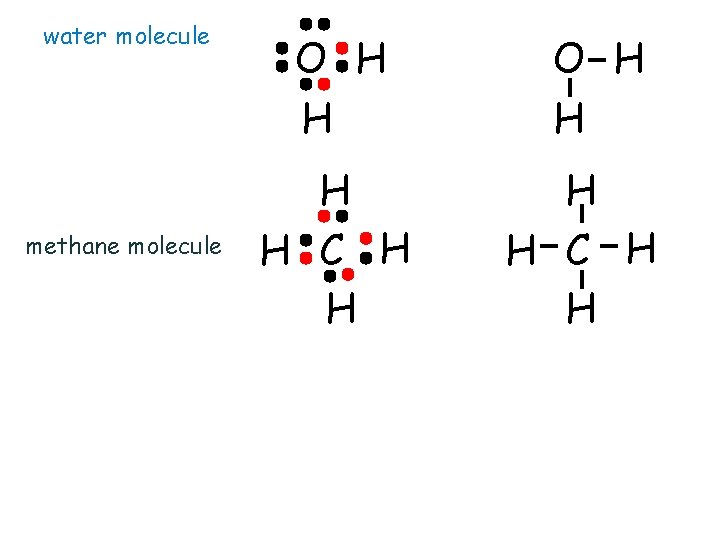
water molecule methane molecule O H H H H C H H
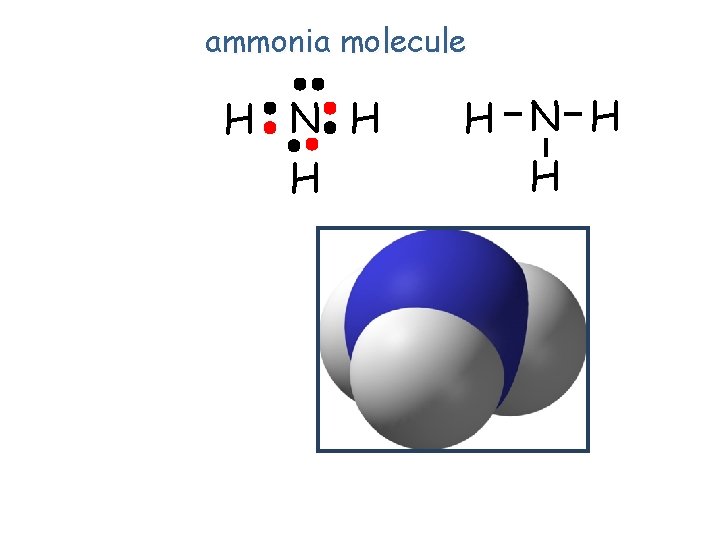
ammonia molecule H N H H
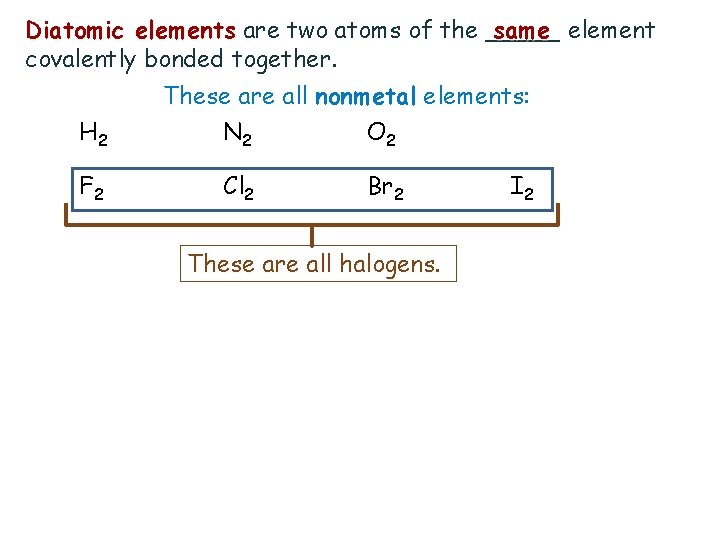
Diatomic elements are two atoms of the _____ same element covalently bonded together. H 2 F 2 These are all nonmetal elements: N 2 O 2 Cl 2 Br 2 These are all halogens. I 2
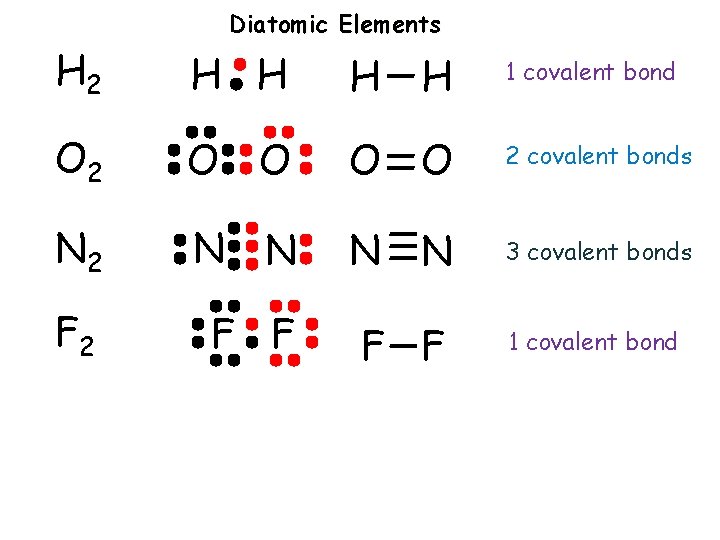
Diatomic Elements H 2 H H 1 covalent bond O 2 O O 2 covalent bonds N 2 N N 3 covalent bonds F 2 F F 1 covalent bond
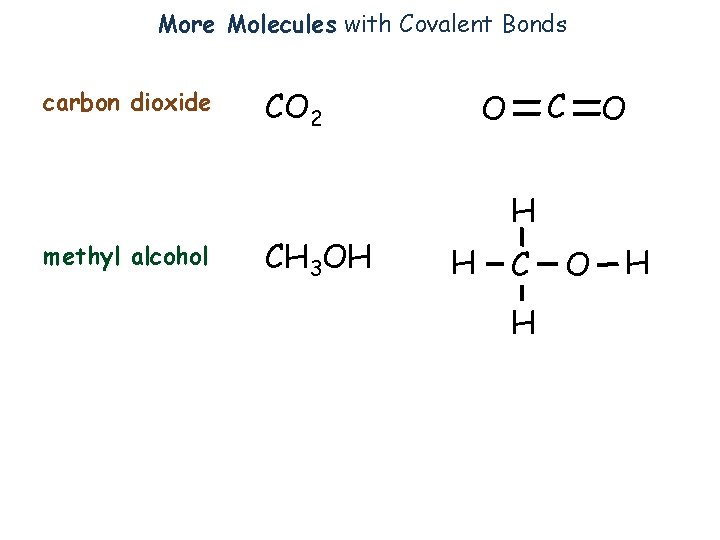
More Molecules with Covalent Bonds carbon dioxide CO 2 C O O H methyl alcohol CH 3 OH H C H O H
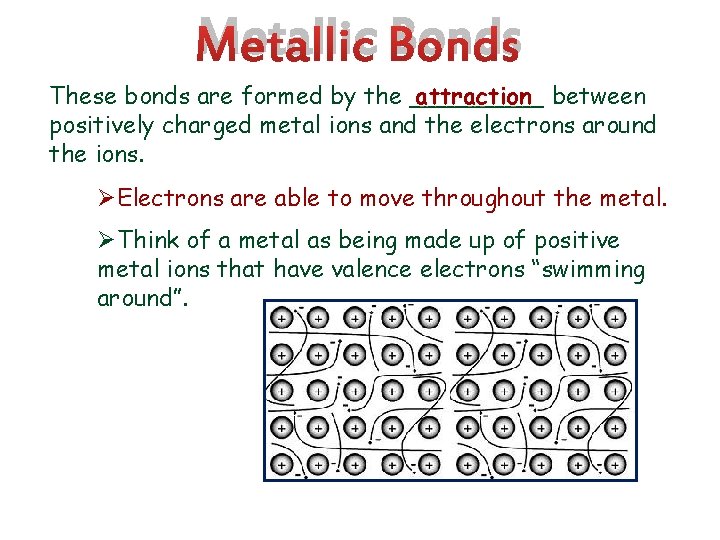
Metallic Bonds These bonds are formed by the _____ attraction between positively charged metal ions and the electrons around the ions. ØElectrons are able to move throughout the metal. ØThink of a metal as being made up of positive metal ions that have valence electrons “swimming around”.
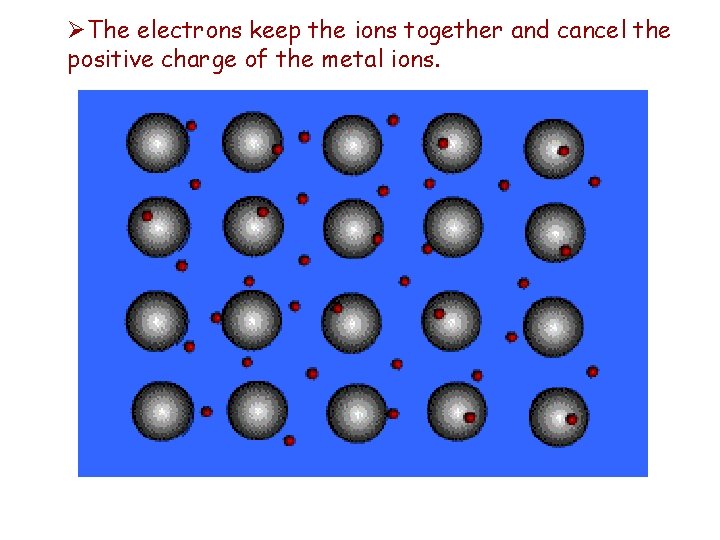
ØThe electrons keep the ions together and cancel the positive charge of the metal ions.
- Slides: 13