Covalent Compounds TAKE OUT YOUR PERIODIC TABLE QUIZ

Covalent Compounds TAKE OUT YOUR PERIODIC TABLE QUIZ TODAY – QUESTIONS?

What is a covalent compound? Covalent Compound: ◦ Is a compound made up of two or more nonmetals that are bonded together - the electrons are shared instead of transferred - the have NO charges
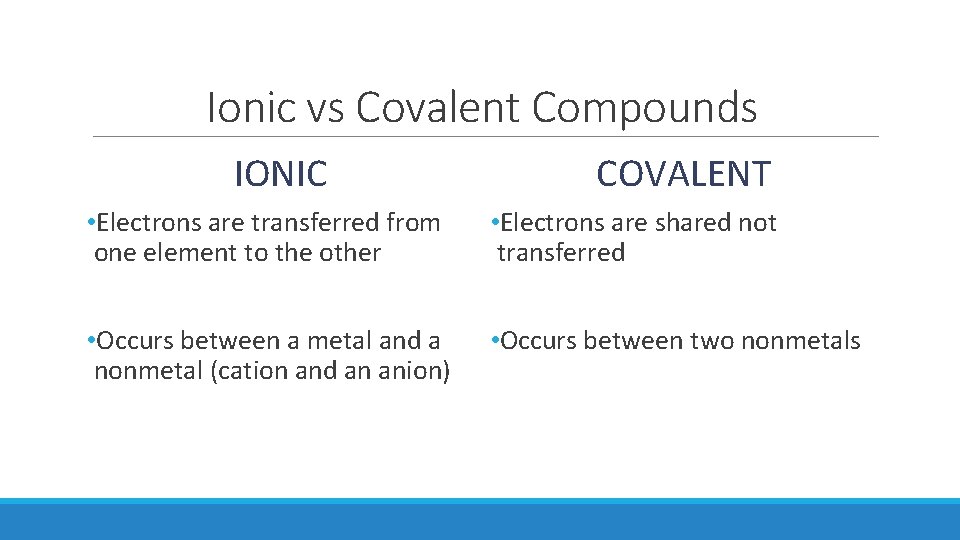
Ionic vs Covalent Compounds IONIC COVALENT • Electrons are transferred from one element to the other • Electrons are shared not transferred • Occurs between a metal and a nonmetal (cation and an anion) • Occurs between two nonmetals
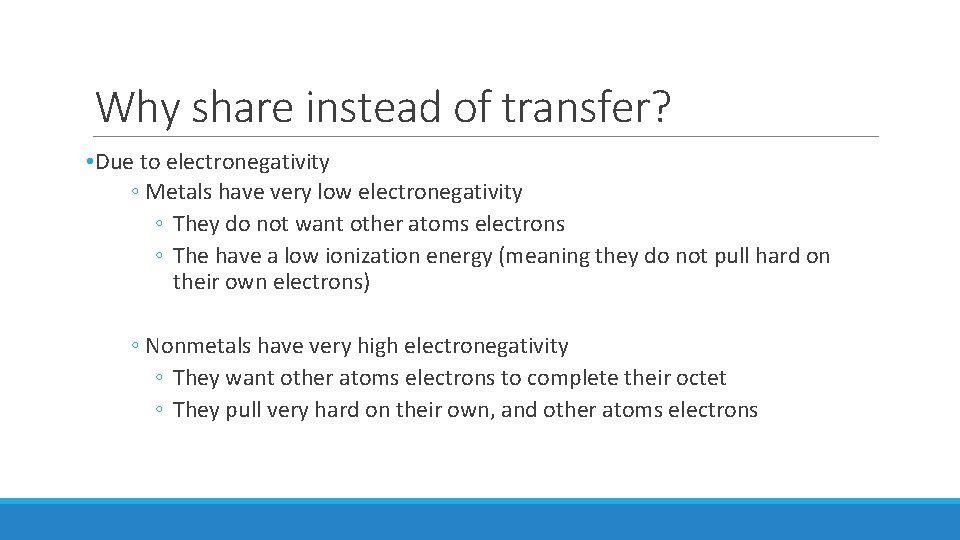
Why share instead of transfer? • Due to electronegativity ◦ Metals have very low electronegativity ◦ They do not want other atoms electrons ◦ The have a low ionization energy (meaning they do not pull hard on their own electrons) ◦ Nonmetals have very high electronegativity ◦ They want other atoms electrons to complete their octet ◦ They pull very hard on their own, and other atoms electrons
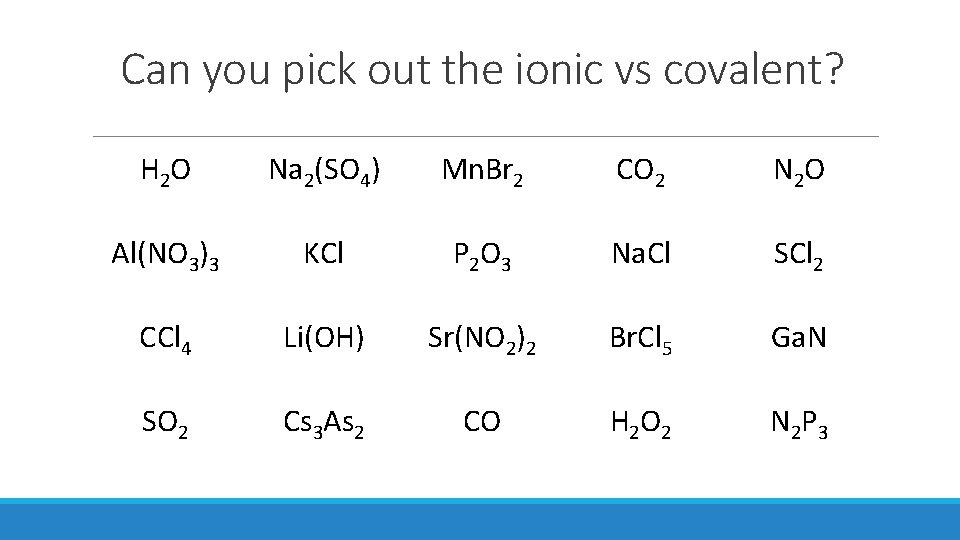
Can you pick out the ionic vs covalent? H 2 O Na 2(SO 4) Mn. Br 2 CO 2 N 2 O Al(NO 3)3 KCl P 2 O 3 Na. Cl SCl 2 CCl 4 Li(OH) Sr(NO 2)2 Br. Cl 5 Ga. N SO 2 Cs 3 As 2 CO H 2 O 2 N 2 P 3
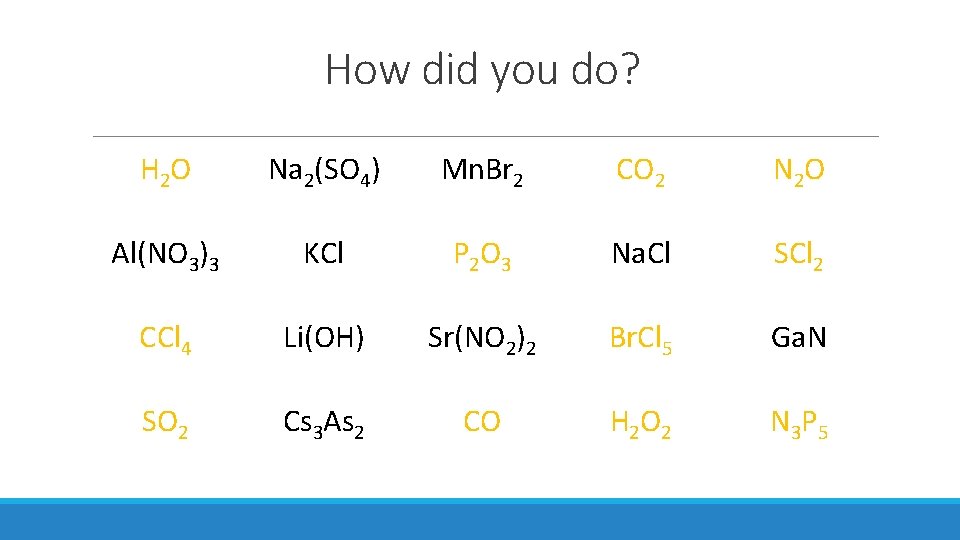
How did you do? H 2 O Na 2(SO 4) Mn. Br 2 CO 2 N 2 O Al(NO 3)3 KCl P 2 O 3 Na. Cl SCl 2 CCl 4 Li(OH) Sr(NO 2)2 Br. Cl 5 Ga. N SO 2 Cs 3 As 2 CO H 2 O 2 N 3 P 5
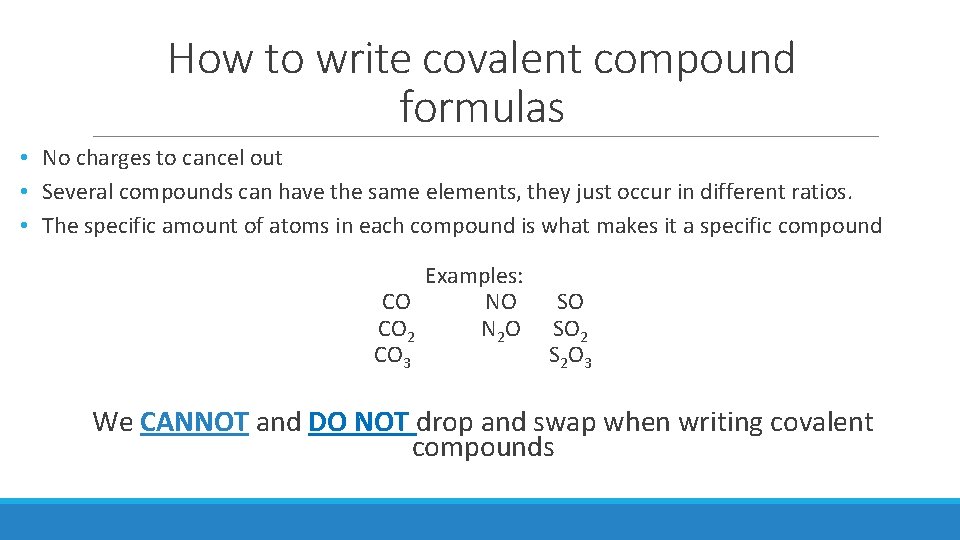
How to write covalent compound formulas • No charges to cancel out • Several compounds can have the same elements, they just occur in different ratios. • The specific amount of atoms in each compound is what makes it a specific compound Examples: CO NO CO 2 N 2 O CO 3 SO SO 2 S 2 O 3 We CANNOT and DO NOT drop and swap when writing covalent compounds
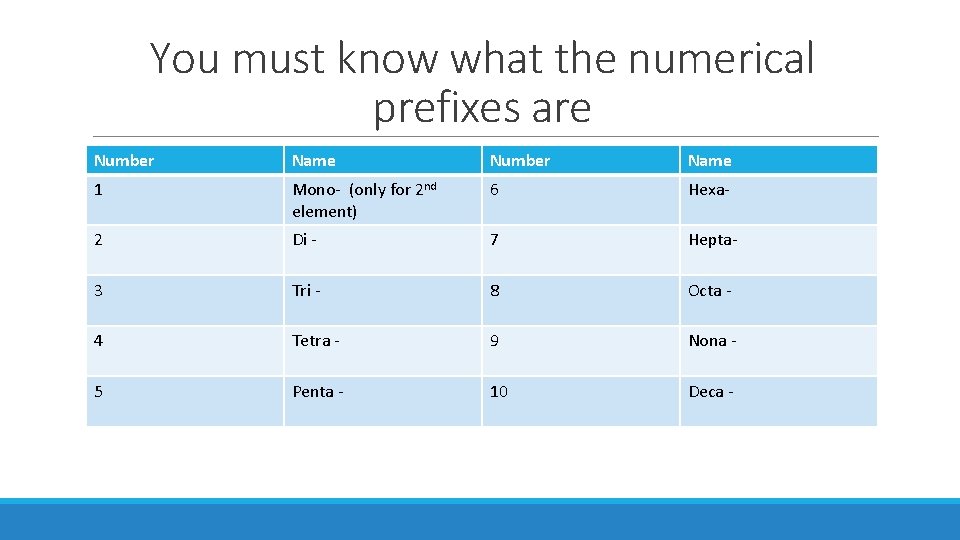
You must know what the numerical prefixes are Number Name 1 Mono- (only for 2 nd element) 6 Hexa- 2 Di - 7 Hepta- 3 Tri - 8 Octa - 4 Tetra - 9 Nona - 5 Penta - 10 Deca -
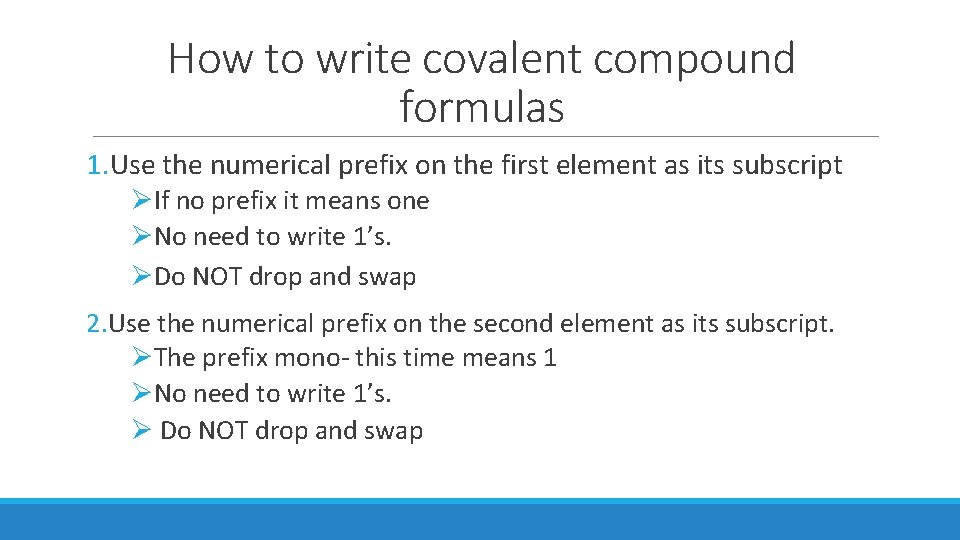
How to write covalent compound formulas 1. Use the numerical prefix on the first element as its subscript ØIf no prefix it means one ØNo need to write 1’s. ØDo NOT drop and swap 2. Use the numerical prefix on the second element as its subscript. ØThe prefix mono- this time means 1 ØNo need to write 1’s. Ø Do NOT drop and swap
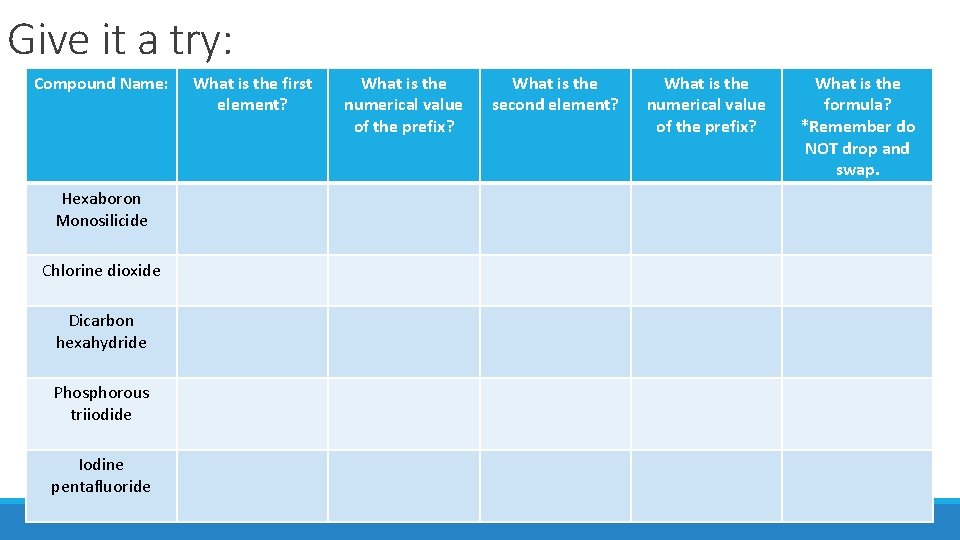
Give it a try: Compound Name: Hexaboron Monosilicide Chlorine dioxide Dicarbon hexahydride Phosphorous triiodide Iodine pentafluoride What is the first element? What is the numerical value of the prefix? What is the second element? What is the numerical value of the prefix? What is the formula? *Remember do NOT drop and swap.
How to name a covalent compound: 1. NEVER simplify these compounds ◦ Example: N 2 O 4 IS NOT NO 2 2. For the 1 st element: Use a prefix for all numbers EXCEPT one and the elements name off the periodic table. ◦ NO 2 (Nitrogen does not need a prefix for this name) ◦ N 2 O (Nitrogen does need the prefix di- to indicate there are two nitrogen atoms) 3. For the 2 nd element: ALWAYS use a prefix 4. For the 2 nd element: Change the ending of the elements name to “-ide”

How to name a covalent compound: C 2 O 6 1. The first element you simply name and add the proper prefix if needed 2. The second element you name with the proper prefix 3. For the second element change the ending to “-ide” Dicarbon Hexaoxide
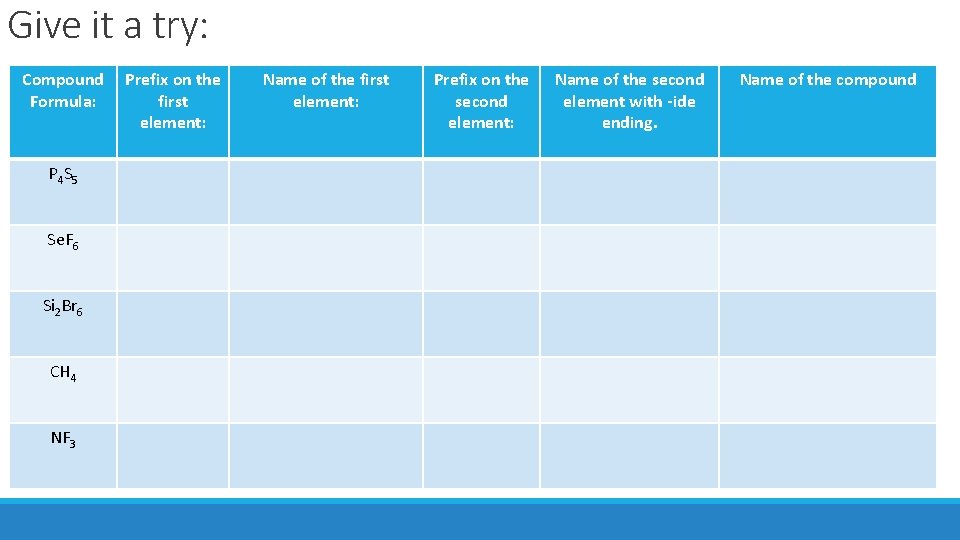
Give it a try: Compound Formula: P 4 S 5 Se. F 6 Si 2 Br 6 CH 4 NF 3 Prefix on the first element: Name of the first element: Prefix on the second element: Name of the second element with -ide ending. Name of the compound
- Slides: 13