Covalent Compounds Lewis Dot Structure polarity molecule shapes








- Slides: 20

Covalent Compounds Lewis Dot Structure, polarity, molecule shapes, forces 1

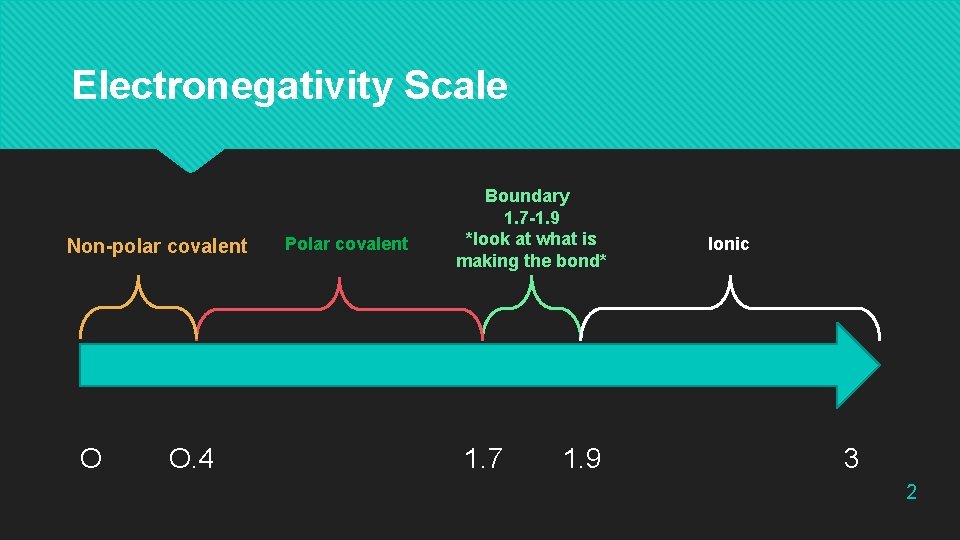
Electronegativity Scale Non-polar covalent O O. 4 Polar covalent Boundary 1. 7 -1. 9 *look at what is making the bond* 1. 7 1. 9 Ionic 3 2

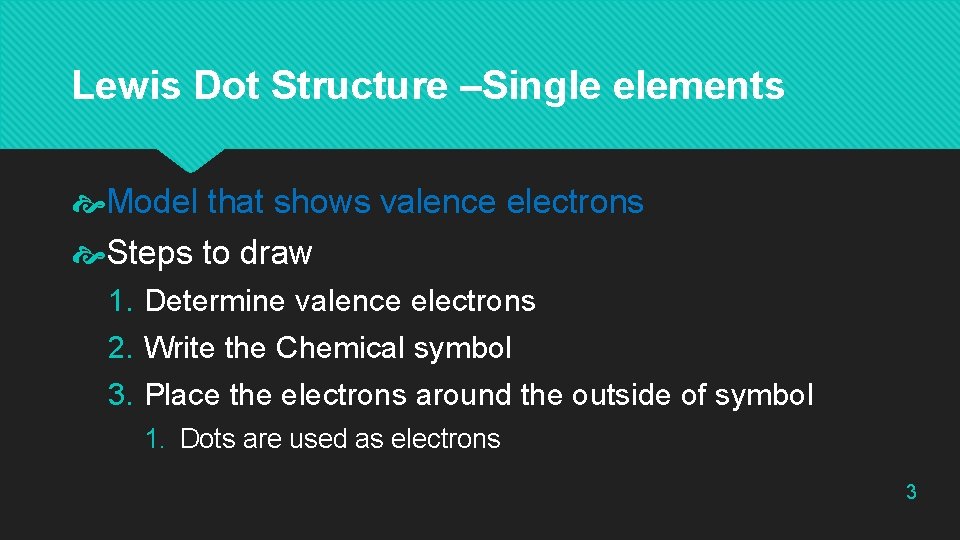
Lewis Dot Structure –Single elements Model that shows valence electrons Steps to draw 1. Determine valence electrons 2. Write the Chemical symbol 3. Place the electrons around the outside of symbol 1. Dots are used as electrons 3

Lets Draw some element Lewis Dot Structures! Chlorine Sodium Carbon Cl: 7 Valence (group 17) Na: 1 Valence (group 1) C: 4 valence (group 14) Na C Cl 4

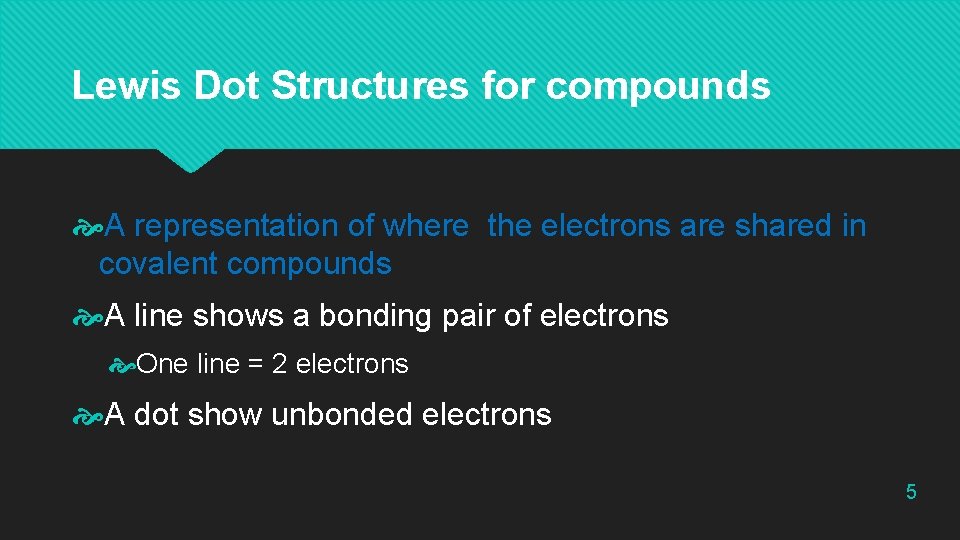
Lewis Dot Structures for compounds A representation of where the electrons are shared in covalent compounds A line shows a bonding pair of electrons One line = 2 electrons A dot show unbonded electrons 5

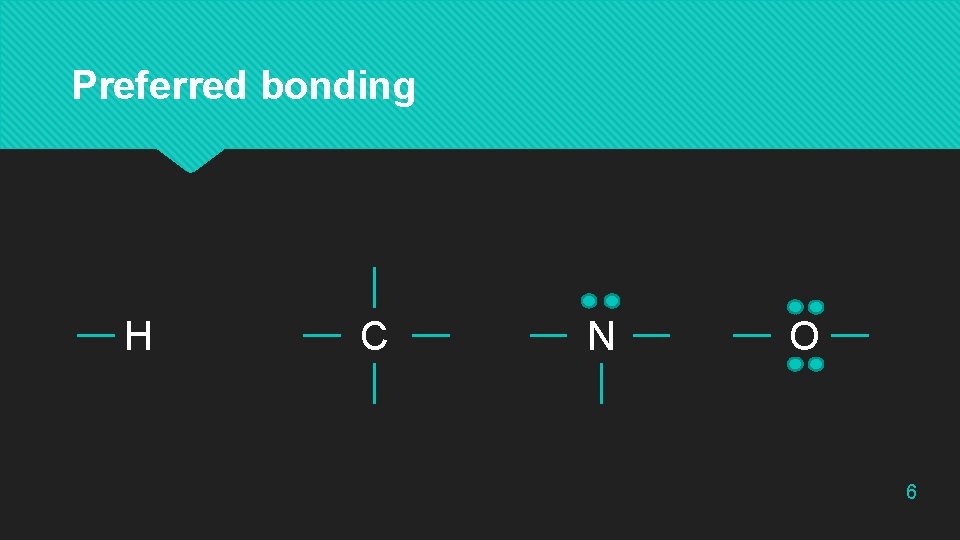
Preferred bonding H C N O 6

Steps to make LDS for compounds 1. Determine valence electrons for all elements 1. Add all the valence electrons together 1. Total for the compound (MAX #) 2. Determine placement of elements 1. Most electro-positive element in center 1. NEVER Hydrogen 3. Place bonds to connect , start with single bonds first 4. Place remaining electrons OR create multiple bond if NEEDED 1. Multiple bonds are needed ONLY when octet cannot be achieved 5. Check for octet 7

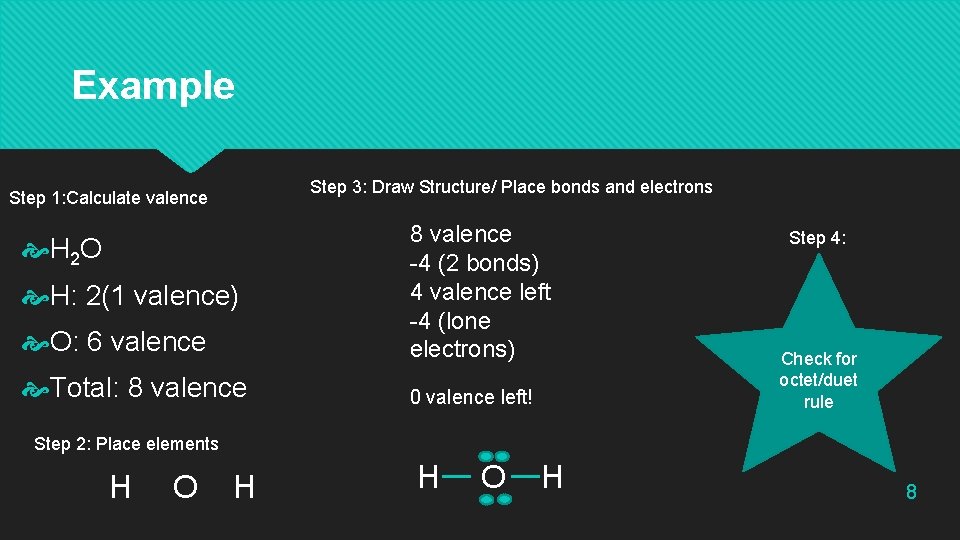
Example Step 3: Draw Structure/ Place bonds and electrons Step 1: Calculate valence O: 6 valence 8 valence -4 (2 bonds) 4 valence left -4 (lone electrons) Total: 8 valence 0 valence left! H 2 O H: 2(1 valence) Step 4: Check for octet/duet rule Step 2: Place elements H O H 8

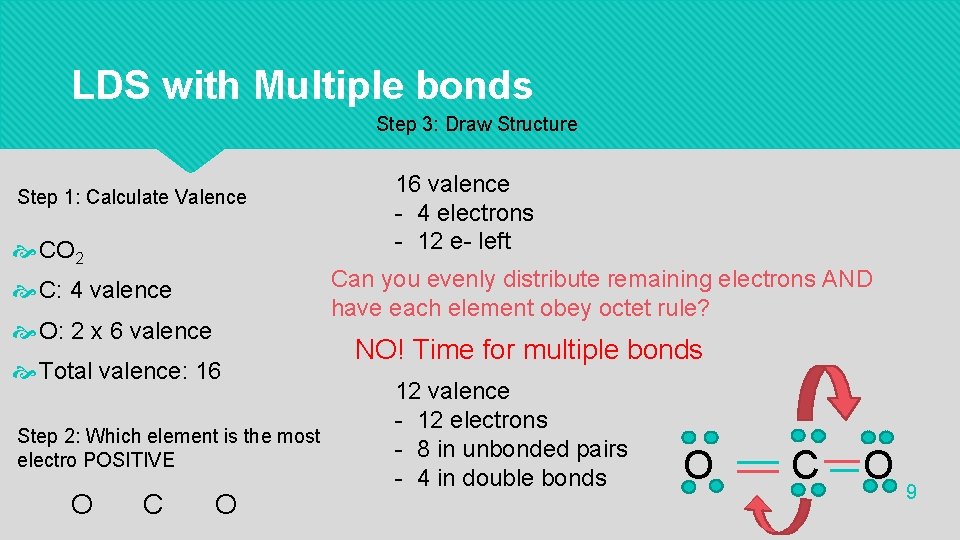
LDS with Multiple bonds Step 3: Draw Structure Step 1: Calculate Valence CO 2 Can you evenly distribute remaining electrons AND have each element obey octet rule? C: 4 valence O: 2 x 6 valence Total valence: 16 Step 2: Which element is the most electro POSITIVE O C 16 valence - 4 electrons - 12 e- left O NO! Time for multiple bonds 12 valence - 12 electrons - 8 in unbonded pairs - 4 in double bonds O C O 9

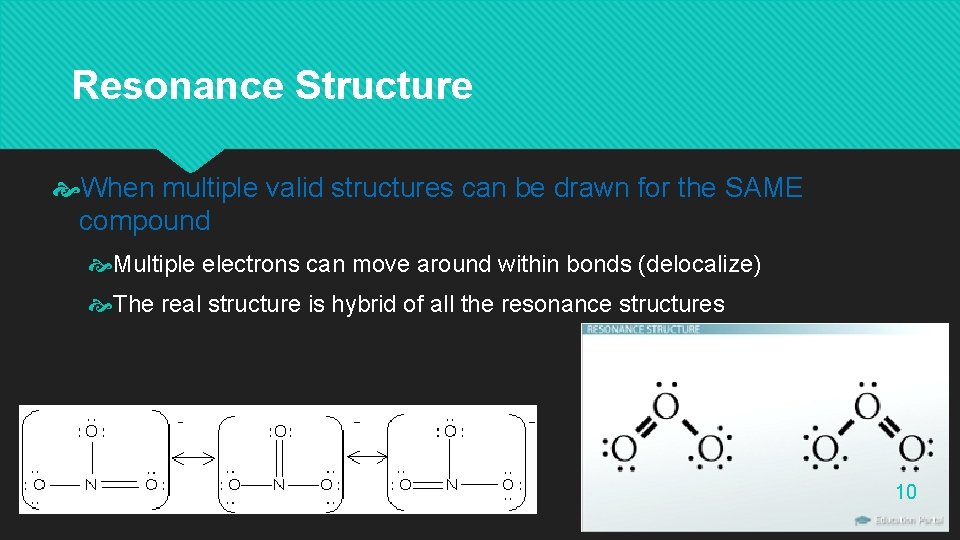
Resonance Structure When multiple valid structures can be drawn for the SAME compound Multiple electrons can move around within bonds (delocalize) The real structure is hybrid of all the resonance structures 10

VSEPR Theory V: Valence S: Shell E: Electron P: Pair R: Repullsion A way to predict the shape and properties of covalent compounds Bonded and unbonded (unpaired) electrons 11

How to predict shapes 1. Draw LSD for the compound 2. Count the number of unpaired and bonded pairs around the CENTRAL ATOM 3. Predict shape 12

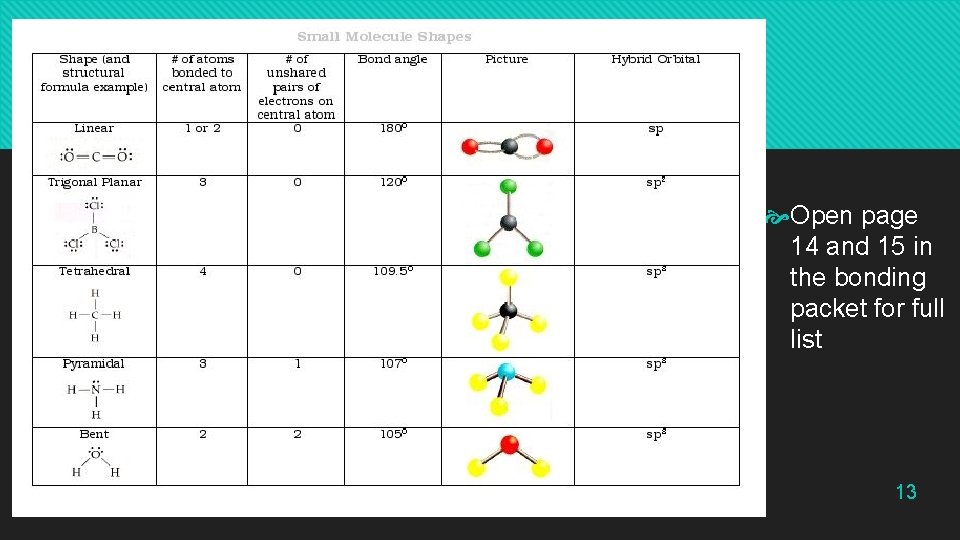
Types of Shape Open page 14 and 15 in the bonding packet for full list 13

Polarity Electronegativity difference within a molecule No difference/slight difference/symmetrical molecule: Non-polar Large difference/ non-symmetrical molecule: Polar Dipole: two poles with difference partial charges 14

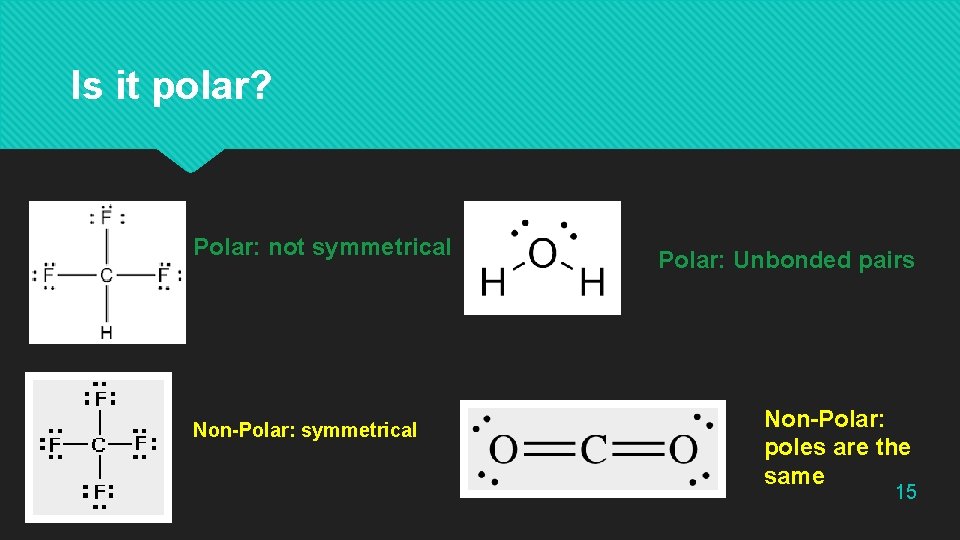
Is it polar? Polar: not symmetrical Non-Polar: symmetrical Polar: Unbonded pairs Non-Polar: poles are the same 15

Intermolecular Forces 1. Van Der Walls, London Dispersion, Induced Dipoles 2. Dipole-Dipole 3. Hydrogen Bonding 16

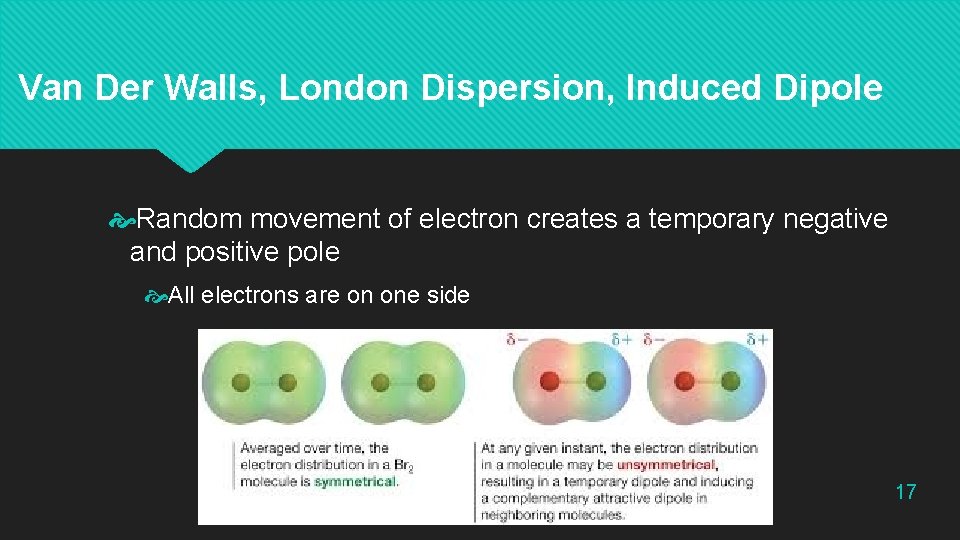
Van Der Walls, London Dispersion, Induced Dipole Random movement of electron creates a temporary negative and positive pole All electrons are on one side 17

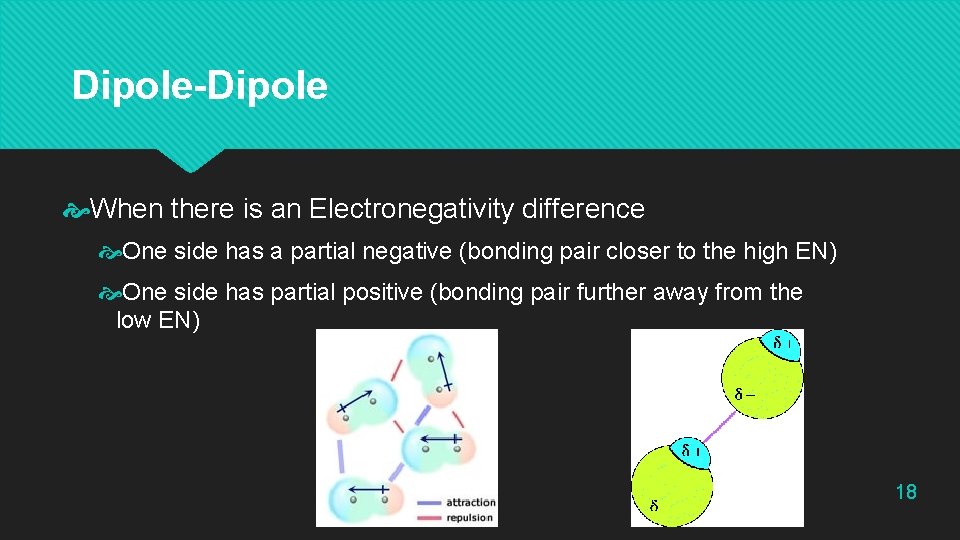
Dipole-Dipole When there is an Electronegativity difference One side has a partial negative (bonding pair closer to the high EN) One side has partial positive (bonding pair further away from the low EN) 18

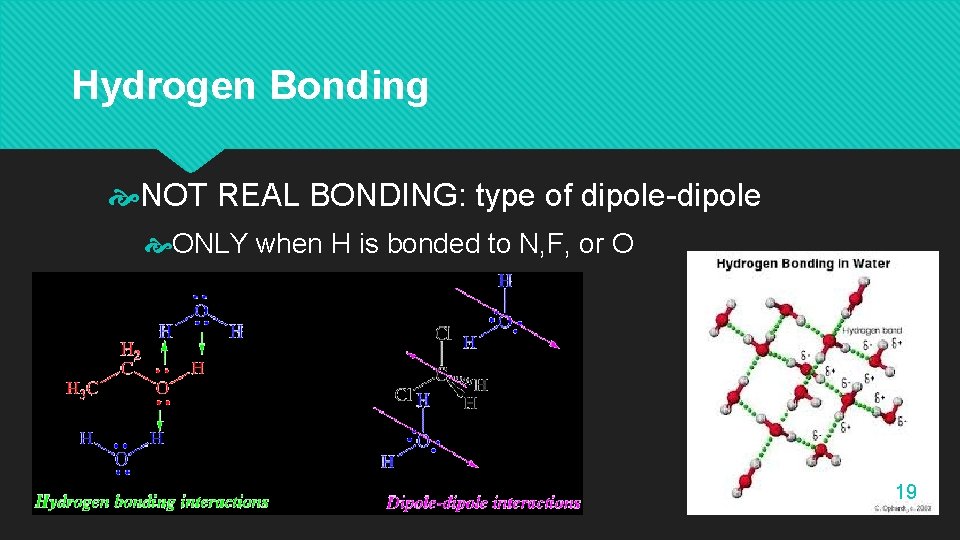
Hydrogen Bonding NOT REAL BONDING: type of dipole-dipole ONLY when H is bonded to N, F, or O 19

What force goes with what? Polar Molecules Dipole-dipole Non-polar molecules London Dispersion Hydrogen bonding ONLY when H is bonded to N, F, or O London Dispersion 20