Covalent Compounds About Covalent Compounds also called molecular
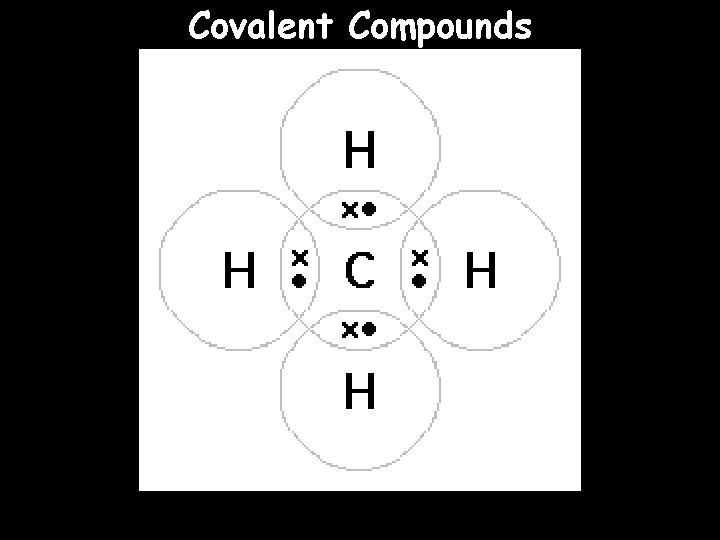
Covalent Compounds

About Covalent Compounds (also called molecular compounds) are any compounds whose atoms are held together by covalent bonds -- remember that covalent bonds are bonds where the electrons are shared between the atoms -- covalent compounds are compounds made of two or more elements that are nonmetals Examples of covalent compounds: -- water -- propane -- oxygen gas

Drawing Covalent Compounds When we draw covalent compounds, we can use our Lewis Structures to show the octet rule (we’ve already practiced this) BUT, a simpler way of drawing involves only showing the electrons involved in the bonding, where we use a line to indicate a shared pair of electrons -- this is called the structural formula For example: Carbon Dioxide (CO 2) Originally O C O Lewis Structure O C O Structural Formula O C O

Practice With Structural Formulas Draw the structural formulas for: Water (H 2 O) Oxygen gas (O 2) Nitrogen gas (N 2)

Properties of Covalent Compounds Low Melting Points Because covalent compounds share electrons and no charges are involved, the pull between the atoms is not quite as strong as in ionic compounds. Therefore, it takes less energy to melt and boil covalent compounds Poor Conductors Since there are no charged particles to move around, covalent compounds do not conduct electricity

Types of Covalent Bonds Even though the atoms share electrons in covalent bonds, the electrons are not always shared equally. There are two ways that electrons can be shared: Polar Covalent Bond – when the electrons are not shared equally between atoms -- one atom has a stronger “pull” on the electrons than the other atom -- the electrons spend more time around the atom with the stronger pull Nonpolar Covalent Bond – when the electrons are shared completely equally

More About Polar Covalent Bonds Polar covalent bonds occur because one atom is more electronegative than the other atom -- this could be due to the number of valence electrons or the number of protons in the nucleus -- the electrons want to spend more time orbiting the more electronegative atom Because of this unequal sharing, one type of atom picks up a slight negative charge and the other type of atom picks up a slight positive charge. . .
Nonpolar Molecules with nonpolar bonds will have NO CHARGE -- usually nonpolar bonds form between two identical types of atoms -- for example the O = O double bond in O 2 -- because neither atom is stronger than the other

Nonpolar vs. Polar How do you know if the bonds are polar covalent or nonpolar or ionic? -- If the bond is between a nonmetal and a metal, it is ionic (or a polyatomic ion) -- If the bond is between two different nonmetals, it is polar covalent -- If the bond is between two of the same type of nonmetal, it is nonpolar covalent In order to figure out whether the molecule itself is polar or nonpolar, you must draw it!

Like Dissolves Like Nonpolar compounds and polar compounds do not mix well together -- this is why oil does not dissolve in water! If you add a molecule, such as soap, that has both nonpolar and polar parts to it, you can get the polar and nonpolar compounds to mix together The nonpolar part of the soap with bind with the oil The polar part of the soap will bind with the water
- Slides: 10