Covalent bonds What keeps you together Covalent bonds

Covalent bonds What keeps you together?
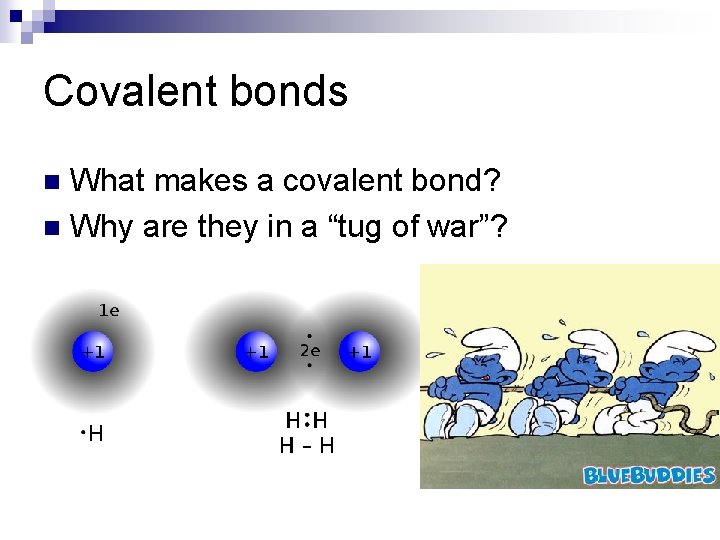
Covalent bonds What makes a covalent bond? n Why are they in a “tug of war”? n
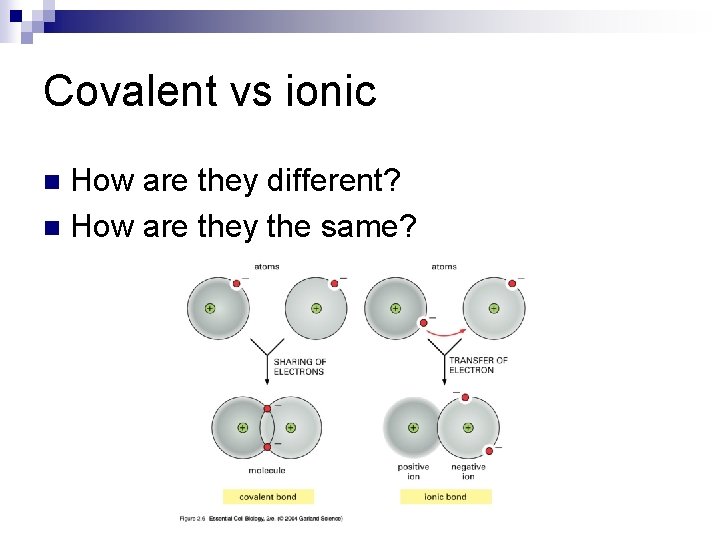
Covalent vs ionic How are they different? n How are they the same? n
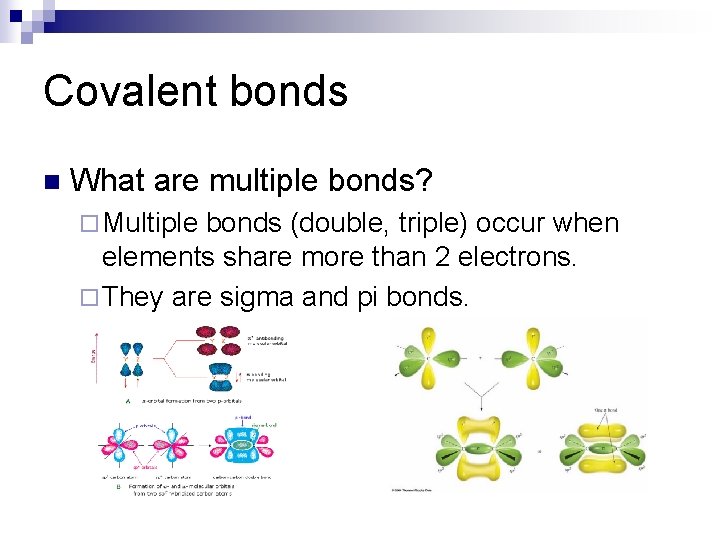
Covalent bonds n What are multiple bonds? ¨ Multiple bonds (double, triple) occur when elements share more than 2 electrons. ¨ They are sigma and pi bonds.
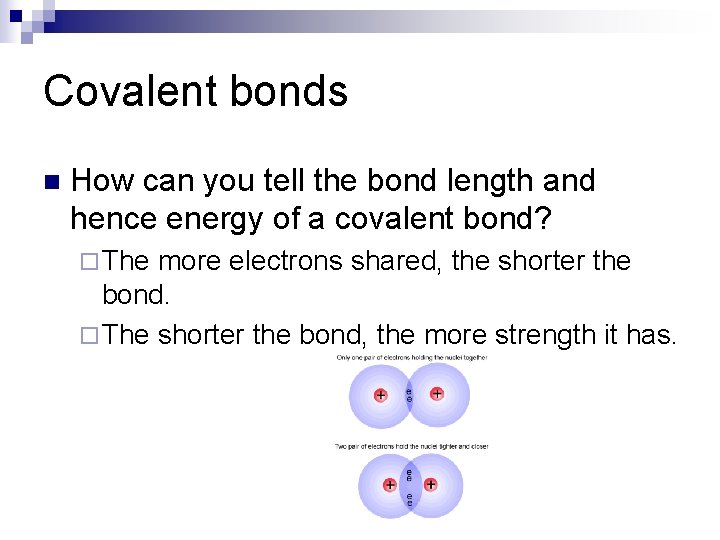
Covalent bonds n How can you tell the bond length and hence energy of a covalent bond? ¨ The more electrons shared, the shorter the bond. ¨ The shorter the bond, the more strength it has.
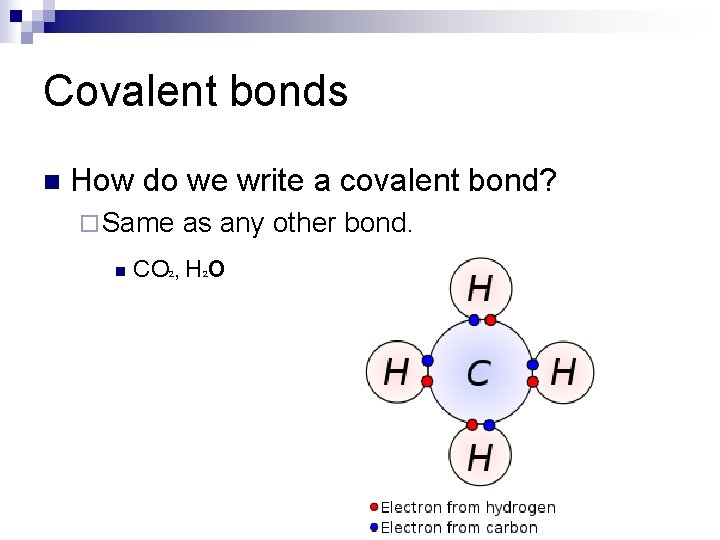
Covalent bonds n How do we write a covalent bond? ¨ Same n as any other bond. CO , H o 2 2

Covalent bonds n How do we name a covalent bond? ¨ The same naming rules apply. ¨ Now you add prefixes to tell the number of atoms of each element. Carbon dioxide n Dihydrogen monoxide n
Covalent bonds n What are the common prefixes? ¨ ¨ ¨ ¨ ¨ 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca
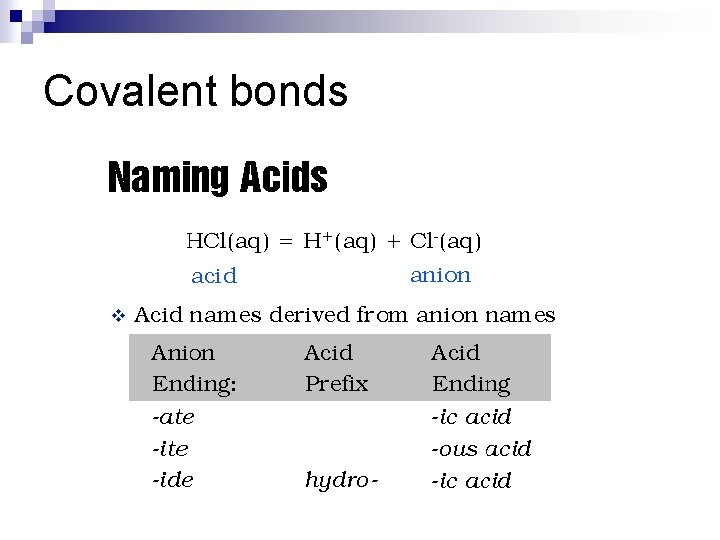
Covalent bonds n How do we name acids? ¨ Acids are hydrogen donors, so the first element in the compound is hydrogen. ¨ If it is hydrogen and a single element the name is hydro-____ic acid. ¨ If it is a polyatomic ion, it is. . .
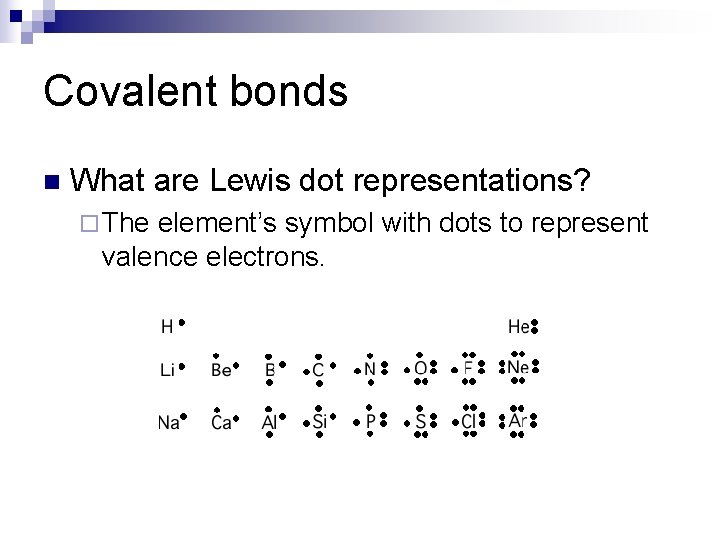
Covalent bonds n What are Lewis dot representations? ¨ The element’s symbol with dots to represent valence electrons.
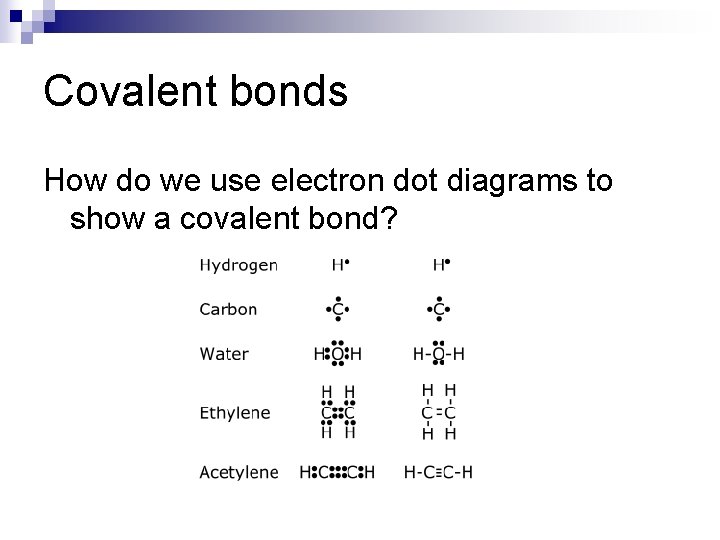
Covalent bonds How do we use electron dot diagrams to show a covalent bond?
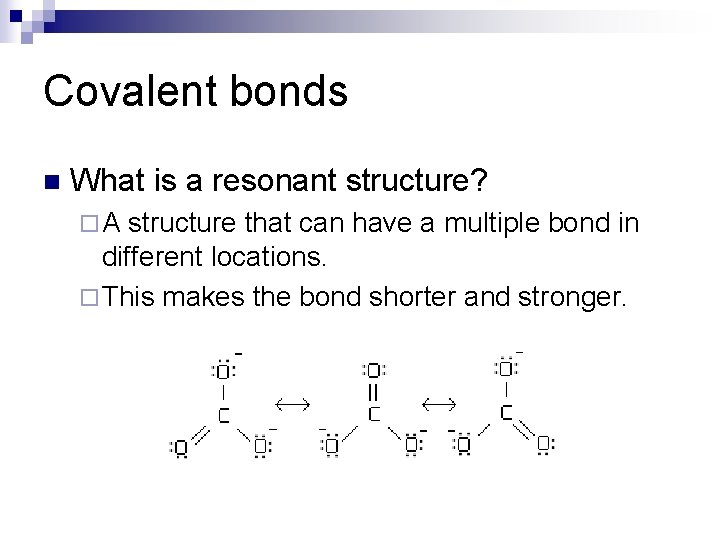
Covalent bonds n What is a resonant structure? ¨A structure that can have a multiple bond in different locations. ¨ This makes the bond shorter and stronger.

Covalent bonds n What are coordinate covalent bonds (dative)? ¨ One element or compound can donate both electrons to a bond.
- Slides: 13