COVALENT BONDS How Bonds Form Electrons are shared
COVALENT BONDS
How Bonds Form Electrons are shared between 2 or more nonmetal atoms. Can also be between a nonmetal and a metalloid. Ex: H 2 O, CO 2, C 6 H 12 O 6 All nonmetal atoms! EN value less than 1. 5
Properties – think sugar! • • • Can be solid, liquid, or gas at room temp. Low melting and boiling points Cannot conduct electricity Insoluble in water Polar Covalent bonds – electrons shared unequally (EN difference 0. 5 – 1. 5) Creates partial + and – ends • Nonpolar Covalent bonds – electrons shared equally (EN difference 0 – 0. 5) • Compounds are called “molecules”
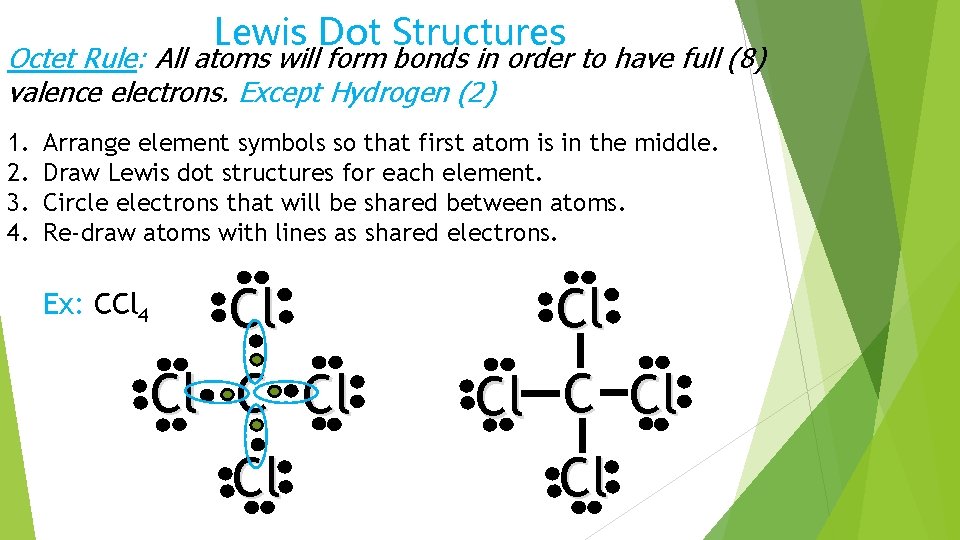
Lewis Dot Structures Octet Rule: All atoms will form bonds in order to have full (8) valence electrons. Except Hydrogen (2) 1. 2. 3. 4. Arrange element symbols so that first atom is in the middle. Draw Lewis dot structures for each element. Circle electrons that will be shared between atoms. Re-draw atoms with lines as shared electrons. Ex: CCl 4 Cl Cl Cl Cl
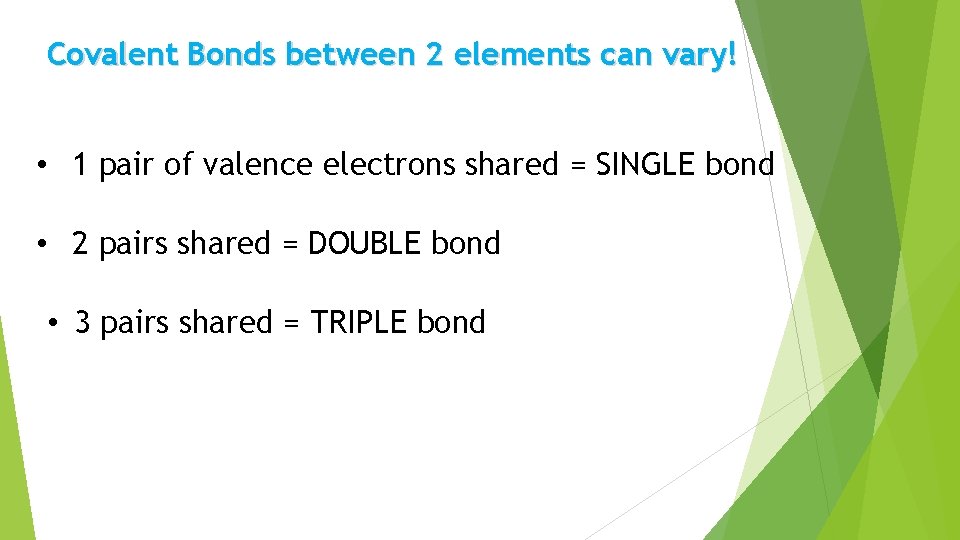
Covalent Bonds between 2 elements can vary! • 1 pair of valence electrons shared = SINGLE bond • 2 pairs shared = DOUBLE bond • 3 pairs shared = TRIPLE bond

Add in these examples under doc camera. H 2 O N 2 NH 3 HF CO 2
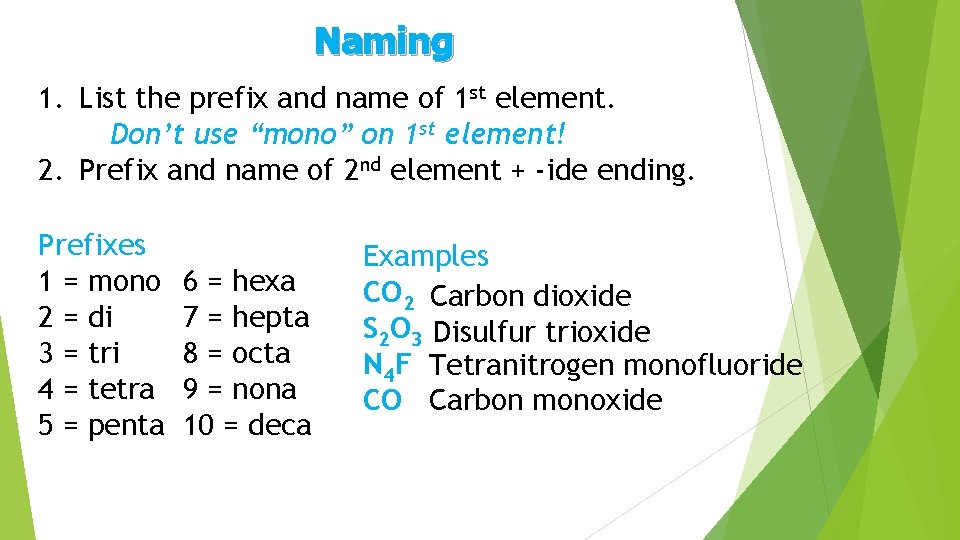
Naming 1. List the prefix and name of 1 st element. Don’t use “mono” on 1 st element! 2. Prefix and name of 2 nd element + -ide ending. Prefixes 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca Examples CO 2 Carbon dioxide S 2 O 3 Disulfur trioxide N 4 F Tetranitrogen monofluoride CO Carbon monoxide
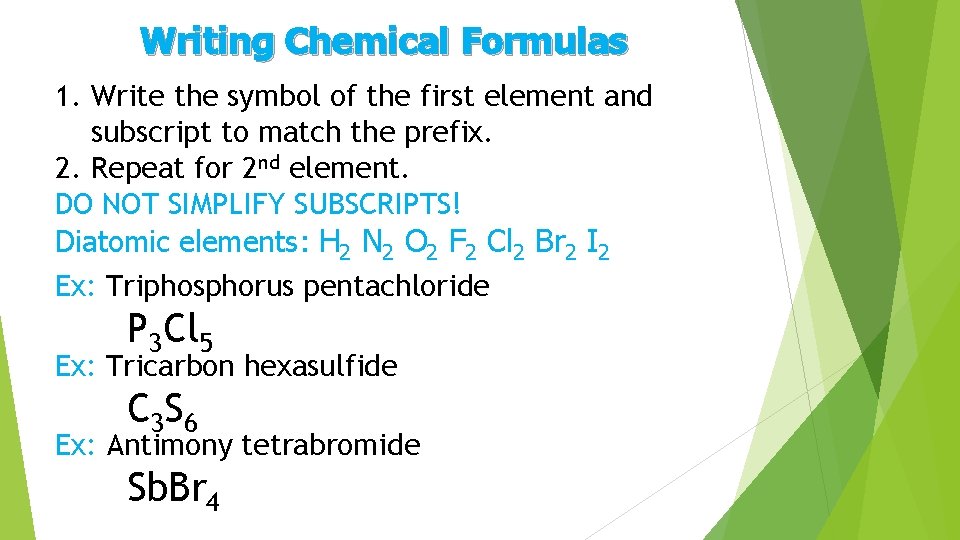
Writing Chemical Formulas 1. Write the symbol of the first element and subscript to match the prefix. 2. Repeat for 2 nd element. DO NOT SIMPLIFY SUBSCRIPTS! Diatomic elements: H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 Ex: Triphosphorus pentachloride P 3 Cl 5 Ex: Tricarbon hexasulfide C 3 S 6 Ex: Antimony tetrabromide Sb. Br 4
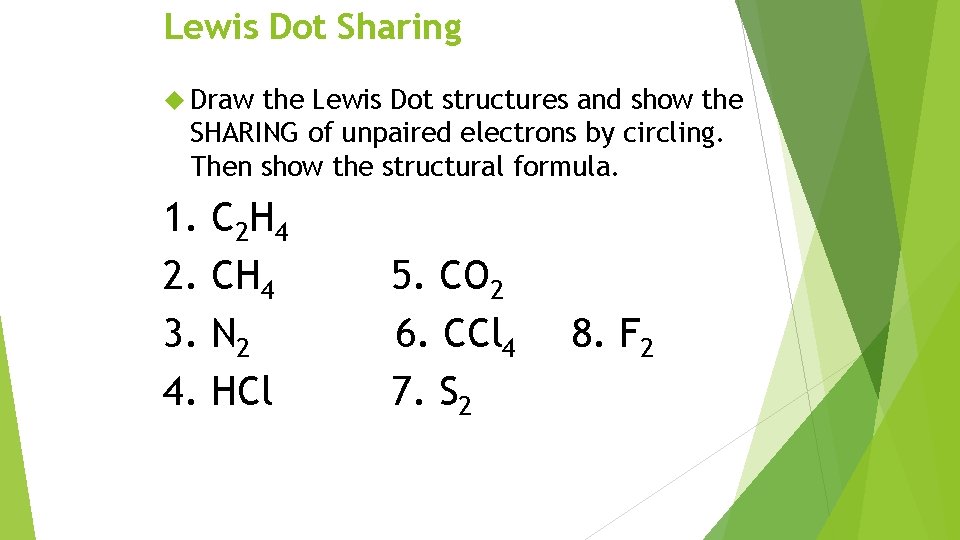
Lewis Dot Sharing Draw the Lewis Dot structures and show the SHARING of unpaired electrons by circling. Then show the structural formula. 1. 2. 3. 4. C 2 H 4 CH 4 N 2 HCl 5. CO 2 6. CCl 4 7. S 2 8. F 2

Recap of Covalent Bonding!
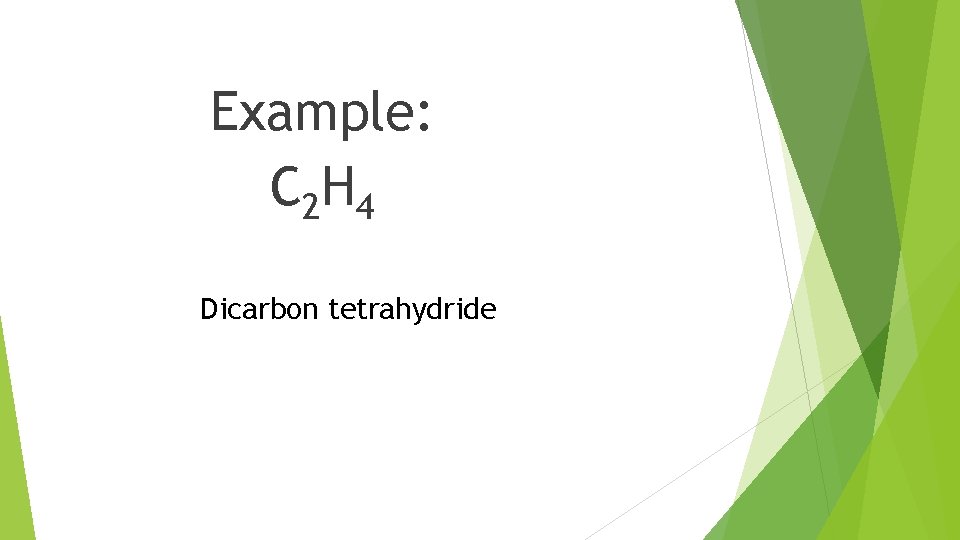
Example: C 2 H 4 Dicarbon tetrahydride

Example: C 2 O 4 Dicarbon tetroxide

Example: Cl 2 O 2 Dichlorine dioxide
- Slides: 13