COVALENT BONDS COVALENT BONDS Covalent Bonds electrons are

COVALENT BONDS

COVALENT BONDS Covalent Bonds – electrons are shared between two atoms • Both atoms “claim” the other’s electron as their own • The number of covalent bonds an atom can form depends on the number of valence electrons it needs to complete its outer shell.
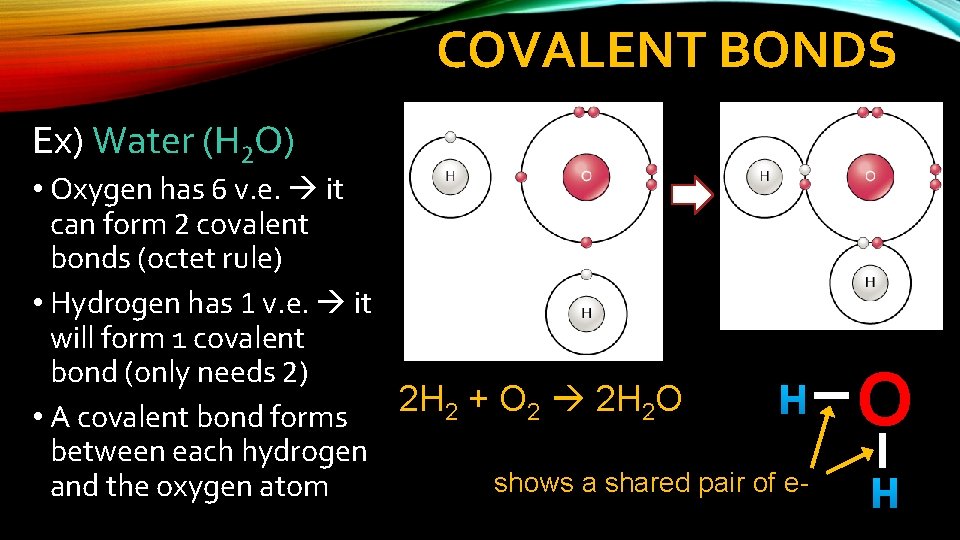
COVALENT BONDS Ex) Water (H 2 O) • Oxygen has 6 v. e. it can form 2 covalent bonds (octet rule) • Hydrogen has 1 v. e. it will form 1 covalent bond (only needs 2) 2 H + O 2 H O H 2 2 2 • A covalent bond forms between each hydrogen shows a shared pair of eand the oxygen atom O H
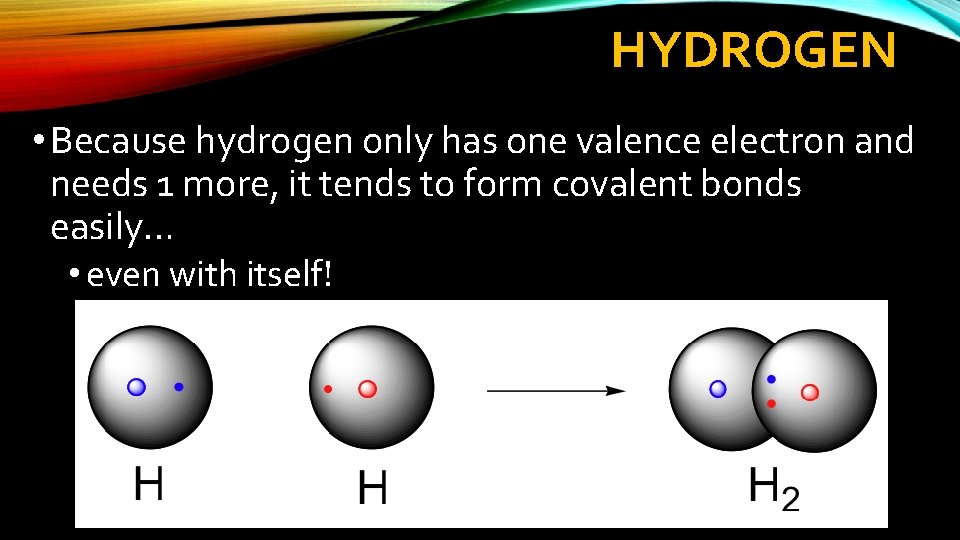
HYDROGEN • Because hydrogen only has one valence electron and needs 1 more, it tends to form covalent bonds easily… • even with itself!
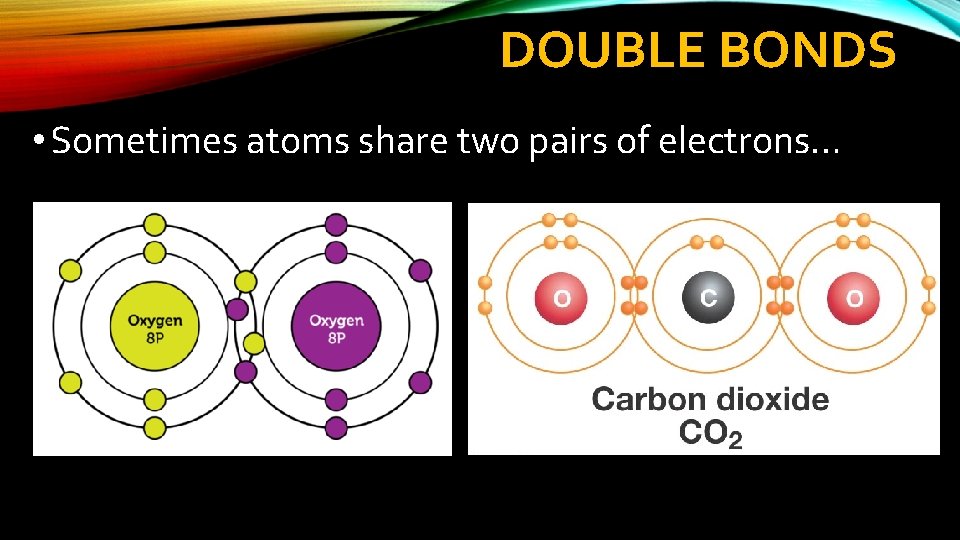
DOUBLE BONDS • Sometimes atoms share two pairs of electrons…
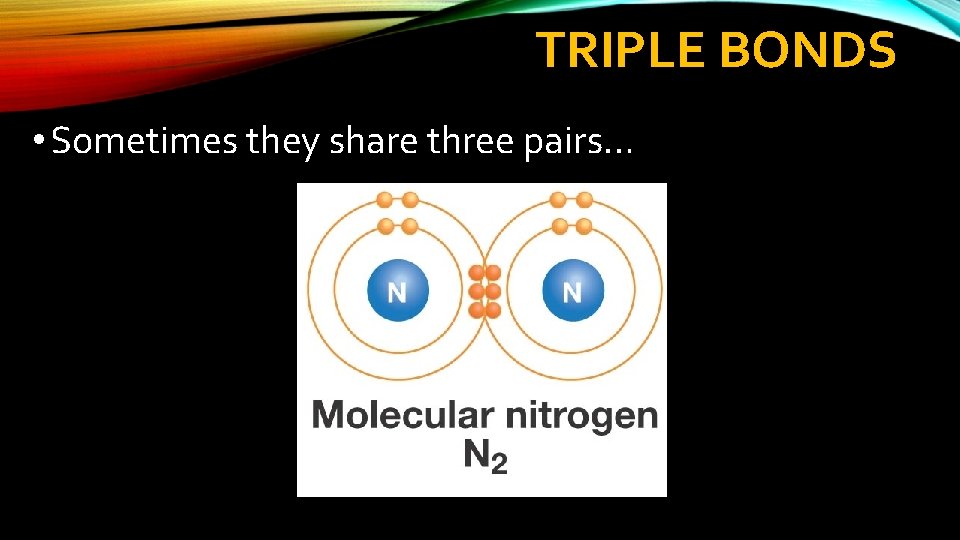
TRIPLE BONDS • Sometimes they share three pairs…
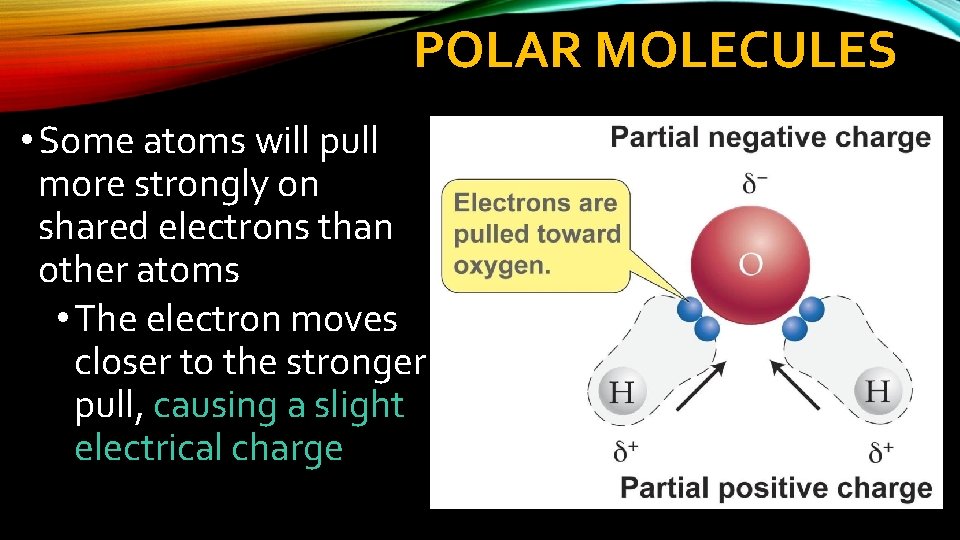
POLAR MOLECULES • Some atoms will pull more strongly on shared electrons than other atoms • The electron moves closer to the stronger pull, causing a slight electrical charge
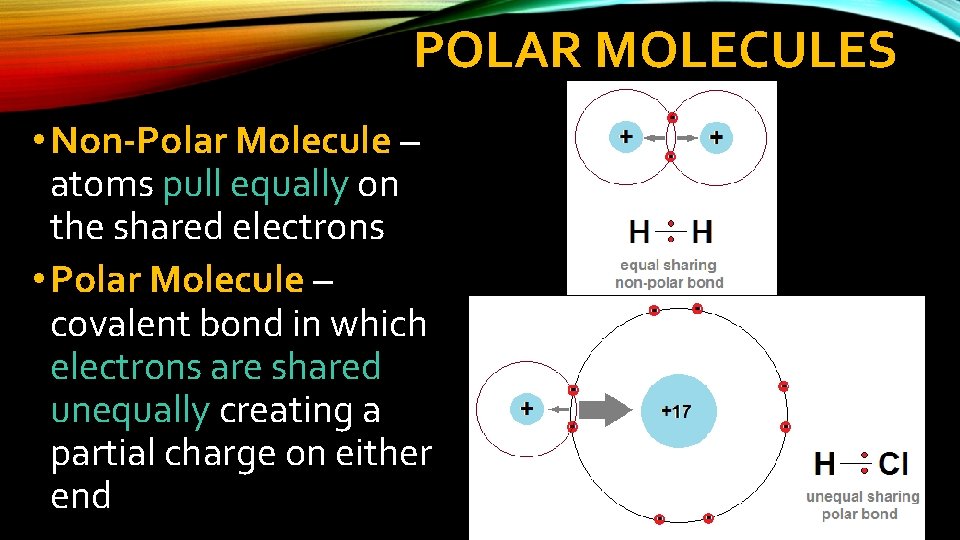
POLAR MOLECULES • Non-Polar Molecule – atoms pull equally on the shared electrons • Polar Molecule – covalent bond in which electrons are shared unequally creating a partial charge on either end
NAMING COVALENT MOLECULES • prefix 1 st element • Note: if 1 st element only has one atom, no prefix is prefix 2 nd element • Prefixes are based on the used number of atoms present: • Examples: • 1 = mono • 2 = di • 3 = tri • 4 = tetra • CO 2 carbon dioxide • H 2 O dihydrogen monoxide • CF 4 carbon tetrafluoride
PROPERTIES OF COVALENT MOLECULES • Low Melting Point • Covalent molecules tend to melt at lower temperatures • Poor Conductors • Most covalent molecules are poor conductors of electricity (electrons do not flow easily)
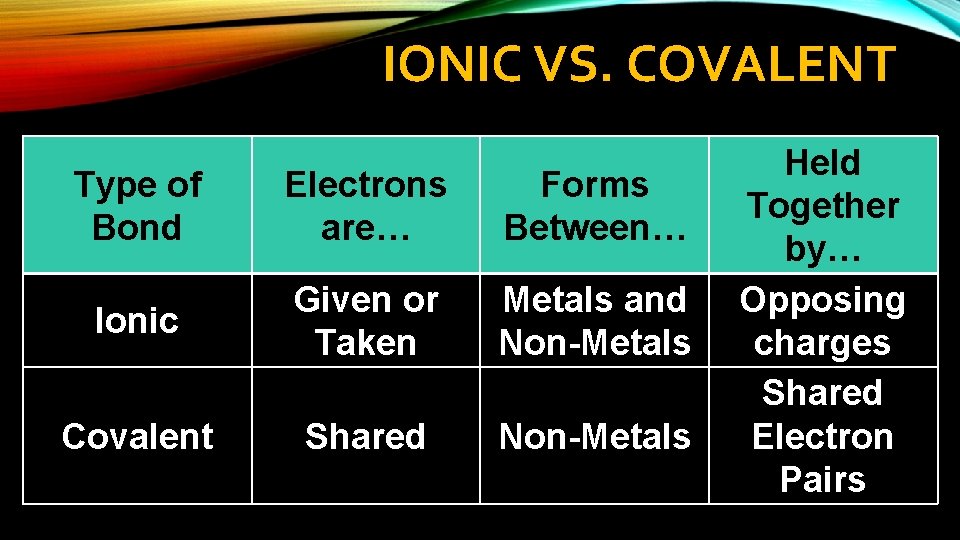
IONIC VS. COVALENT Type of Bond Electrons are… Forms Between… Ionic Given or Taken Metals and Non-Metals Covalent Shared Non-Metals Held Together by… Opposing charges Shared Electron Pairs
- Slides: 11