Covalent Bonds COVALENT BOND bond formed by the

Covalent Bonds
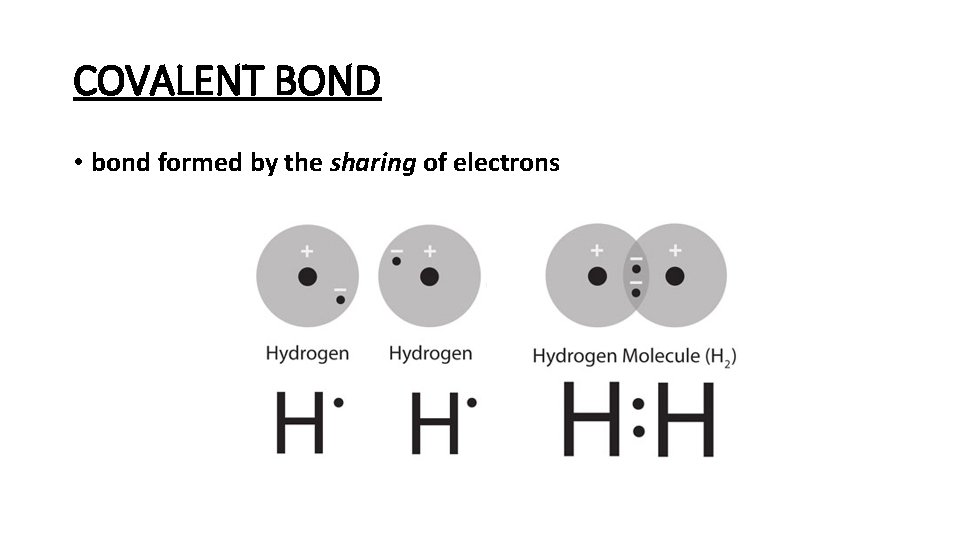
COVALENT BOND • bond formed by the sharing of electrons
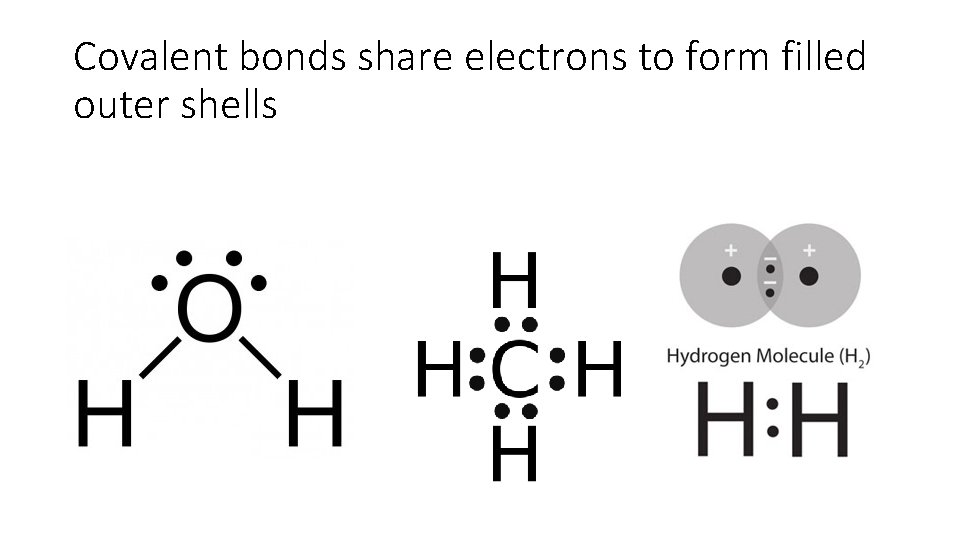
Covalent bonds share electrons to form filled outer shells

Covalent bonds • Between nonmetallic elements • They share electrons because they both want electrons and have similar electronegativity • Hydrogen electronegativity = 2. 1 • Carbon negativity = 2. 5 • They share electrons to form methane CH 4
Covalent characteristics • They have low melting and boiling points • They are most commonly found as liquids and gases at room temperature • They don’t conduct electricity • They are poor heat conductors

Covalent characteristics – low melting points The Covalent bond within molecules is very strong as it is the attraction of a negative electron to a positive nucleus. The attractive forces between covalent molecules is weak and so they don’t stick to together This is why they have low melting points and a regularly gases
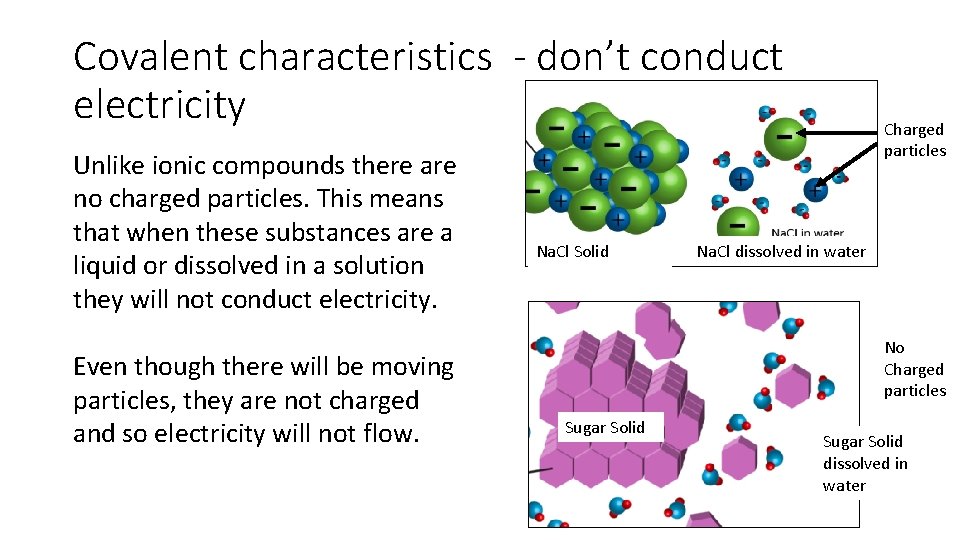
Covalent characteristics - don’t conduct electricity Unlike ionic compounds there are no charged particles. This means that when these substances are a liquid or dissolved in a solution they will not conduct electricity. Even though there will be moving particles, they are not charged and so electricity will not flow. Na. Cl Solid Charged particles Na. Cl dissolved in water No Charged particles Sugar Solid dissolved in water

Covalent characteristics – don’t have good thermal conductivity Lack of free electrons which carry heat energy well. Weak intermolecular forces mean they are often gases and liquids which don’t conduct heat as well as solids.

Naming Covalent compounds Rules • 1. The first element is named first, using the elements name. • 2. Second element is named as an Anion (suffix "-ide") • 3. Prefixes are used to denote the number of atoms • 4. "Mono" is not used to name the first element

Electron dot diagrams for covalent compounds H 2 O Remember it is sharing electrons Atoms share electrons to obtain a filled outer shell No movement of electrons from one atom to another No ions formed No charges needed. Oxygen has 6 VE It wants 8 Hydrogen has 1 VE It wants 2
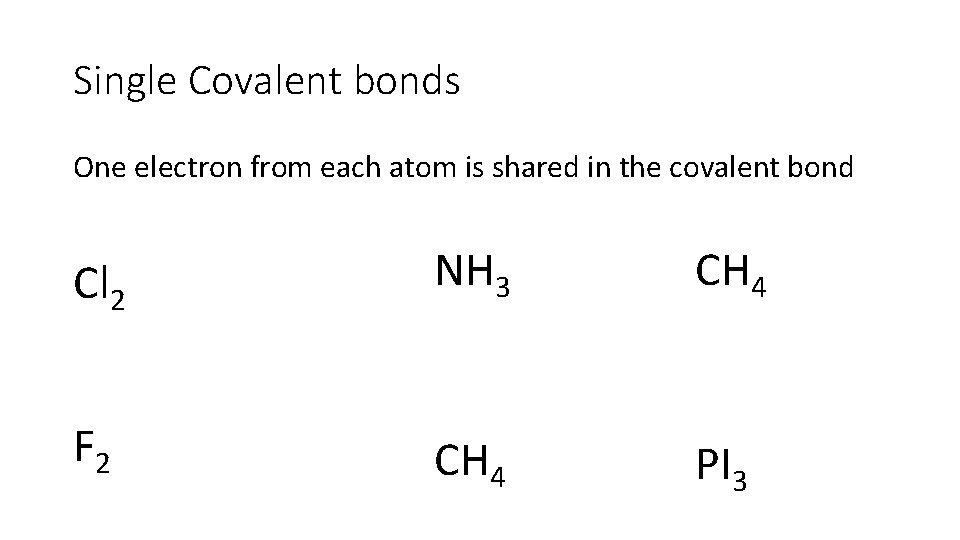
Single Covalent bonds One electron from each atom is shared in the covalent bond Cl 2 NH 3 CH 4 F 2 CH 4 PI 3

Double Covalent bonds Two electrons from each atom are shared in the covalent bond. O 2 CO 2 SO 2

Triple Covalent bonds Three electrons from each atom are shared in the covalent bond. N 2 CO C 2 H 2
- Slides: 13