Covalent Bonds CHAPTER 9 Covalent Bonds Atoms bond

Covalent Bonds CHAPTER 9

Covalent Bonds Atoms bond to other atoms to achieve a stable valence e- configuration (8) Remember: When ionic bonds are formed, eare transferred Covalent Bonds Atoms share e- to achieve stable configuration Occurs between nonmetals-nonmetals
Covalent Bonds Molecule Compound of 2 or more nonmetals bonded covalently Must Know: Hydrogen (H 2), nitrogen (N 2), oxygen (O 2), fluorine (F 2), chlorine (Cl 2), bromine (Br 2), & iodine (I 2) Occur in nature diatomically (2 atoms) b/c the diatomic molecule is more stable than single atom

Single Covalent Bonds When 2 atoms share a pair of e-, it forms a single bond Lewis Structures Use electron-dot diagrams to show e- are arranged in molecules H 2 Cl 2 F 2 H 2 O NH 3 CH 4
Multiple Covalent Bonds When atoms share more than 1 pair of e 2 pairs = double bond 3 pairs = triple bond O 2 N 2 Multiple bonds are stronger than single bonds Single < double< triple
Covalent Bonds Energy must be added in order to break a chemical bond More energy is needed to break a triple bond than a single bond Energy is released when new bonds form Bonded atoms usually more stable than non- bonded atoms This releases energy

Classwork Pg. 244 # 1 -5 Pg. 247 # 7 -10
Naming Binary Molecules Molecular compounds made of 2 different nonmetals Do not contain metals or ions Follow these rules when naming: Step 1: Name 1 st element using the entire element name Step 2: Name 2 nd element changing the end to –ide Step 3: Use prefix indicate the # of atoms of each element present in the compound Table 9 -1, Pg. 248
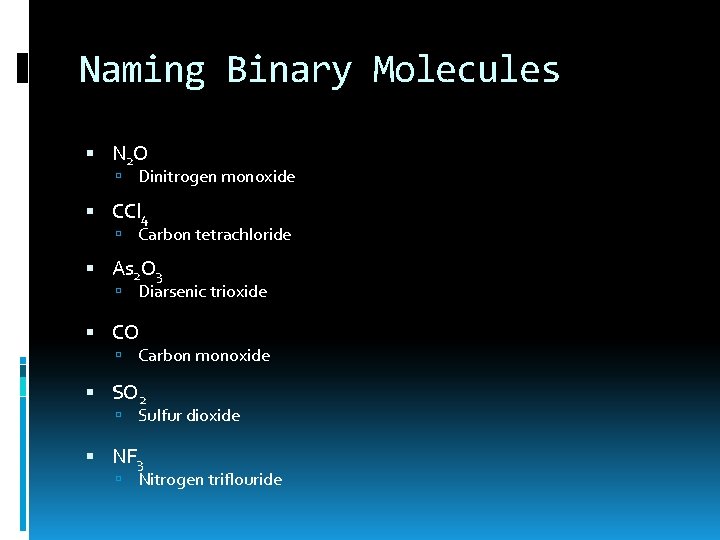
Naming Binary Molecules N 2 O Dinitrogen monoxide CCl 4 Carbon tetrachloride As 2 O 3 Diarsenic trioxide CO Carbon monoxide SO 2 Sulfur dioxide NF 3 Nitrogen triflouride

Naming Acids When some molecules are dissolved in water, they are acidic Named as acids Produces H ions in solution In order to be an acid, it must separate into H ion & some other element HCl separates into H+ and Cl- ions in water Is it an acid? YES When naming:

Naming Acids Step 1: Use prefix hydro- to name the H part of molecule Step 2: Use the name of the 2 nd element and replace the end w/ -ic Step 3: Throw on the acid at the end Name HBr Hydrobromic acid

Naming Acids Name the following: HI Hydroiodic acid HF Hydroflouric acid

HOMEWORK Pg. 251 # 23, 24, 27 a, c, d, e, 28 a
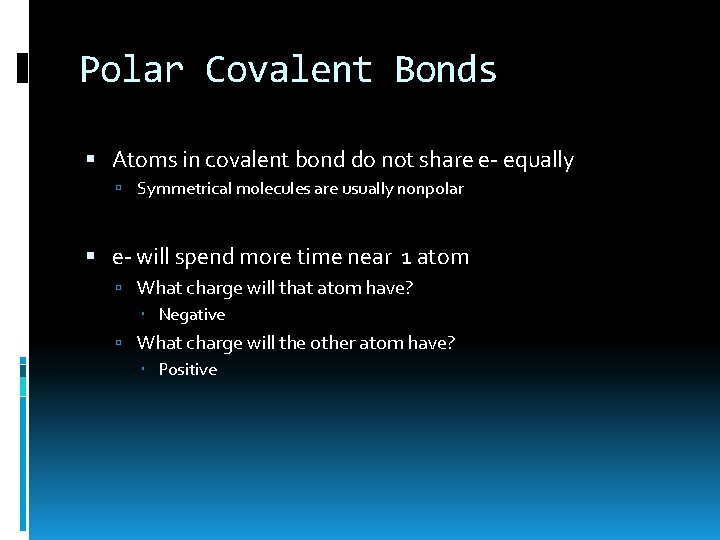
Polar Covalent Bonds Atoms in covalent bond do not share e- equally Symmetrical molecules are usually nonpolar e- will spend more time near 1 atom What charge will that atom have? Negative What charge will the other atom have? Positive

Properties of Covalent Bonds Polar molecules dissolve in other polar molecules Water is polar
- Slides: 15