COVALENT BONDING WHERE ELECTRONS ARE SHARED AND ATOMS

COVALENT BONDING WHERE ELECTRONS ARE SHARED AND ATOMS REMAIN NEUTRAL
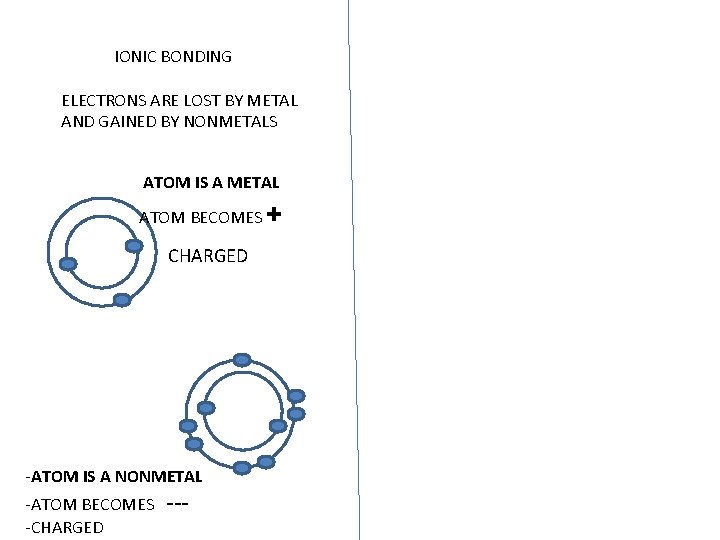
IONIC BONDING ELECTRONS ARE LOST BY METAL AND GAINED BY NONMETALS ATOM IS A METAL ATOM BECOMES CHARGED -ATOM IS A NONMETAL -ATOM BECOMES ---CHARGED +

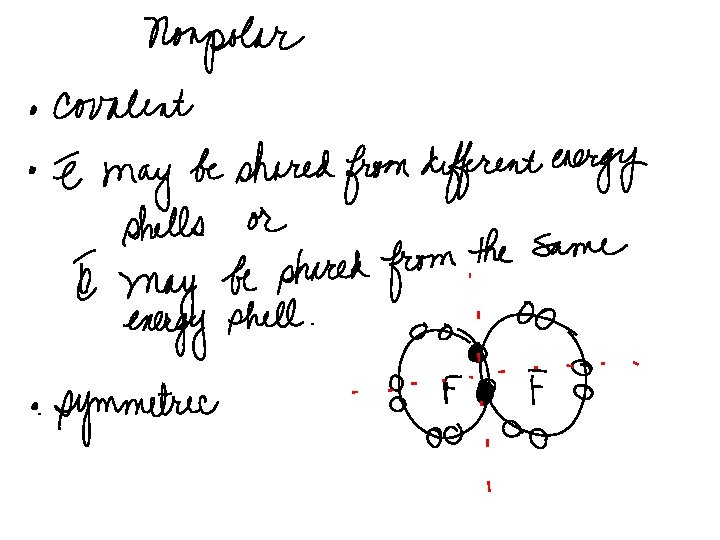
COVALENT BONDING ØSHARING OF ELECTRONS ØATOMS REMAIN NEUTRAL ØOCCURS BETWEEN: v 2 NONMETALS v. A NONMETAL AND A METALLOID v. HYDROGEN AND ALL NONMETALS ØPRODUCES WEAK BONDS ØPRODUCES COMPOUNDS THAT HAVE LOW MELTING POINTS ØALL ORGANIC COMPOUNDS ARE COVALENT

COVALENT BONDINGBETWEEN Ø 2 NONMETALS Nonmetals, because they have more than 4 valence electrons and especially those that have small atomic radii, will bond with themselves in order to become stable and exist at a lower energy state. These atoms will be called DIATOMS. There are 7 diatoms That you will need to memorize. They are F, Cl, Br, I, O, N, H.
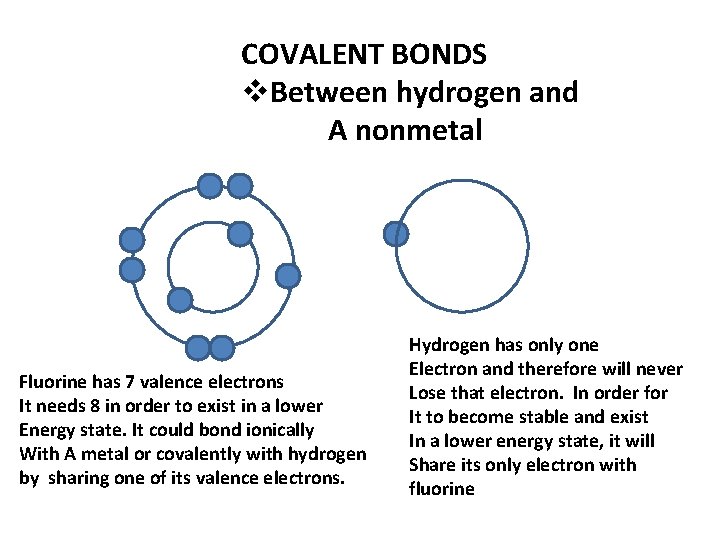
COVALENT BONDS v. Between hydrogen and A nonmetal Fluorine has 7 valence electrons It needs 8 in order to exist in a lower Energy state. It could bond ionically With A metal or covalently with hydrogen by sharing one of its valence electrons. Hydrogen has only one Electron and therefore will never Lose that electron. In order for It to become stable and exist In a lower energy state, it will Share its only electron with fluorine
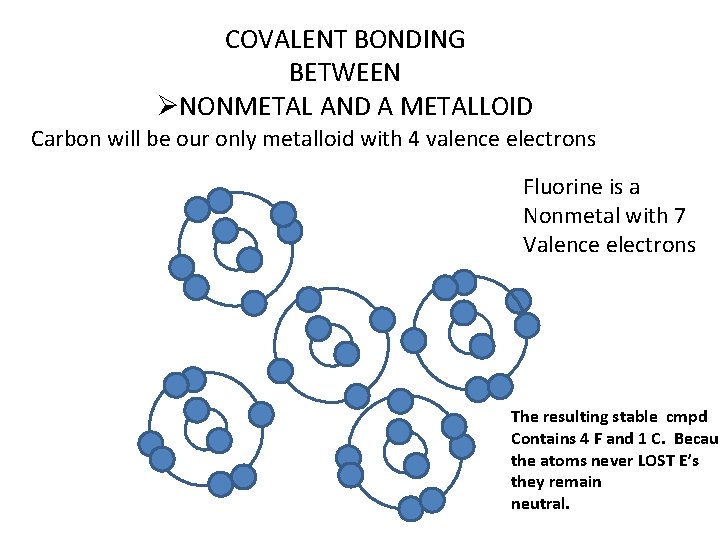
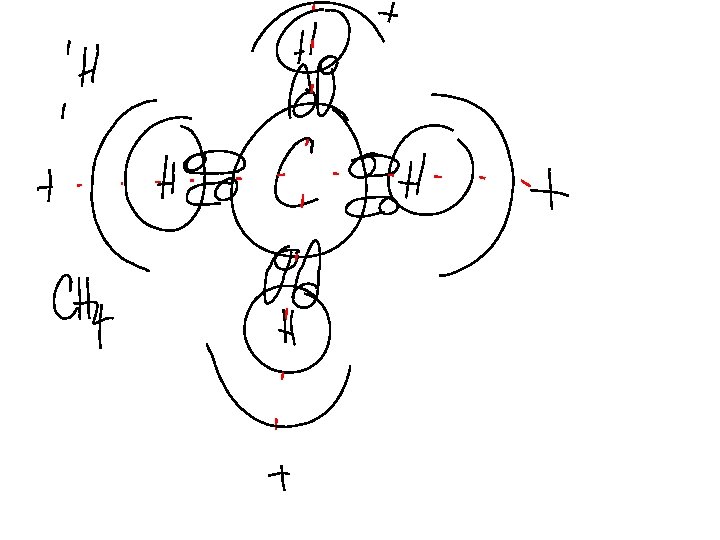
COVALENT BONDING BETWEEN ØNONMETAL AND A METALLOID Carbon will be our only metalloid with 4 valence electrons Fluorine is a Nonmetal with 7 Valence electrons The resulting stable cmpd Contains 4 F and 1 C. Becaus the atoms never LOST E’s they remain neutral.
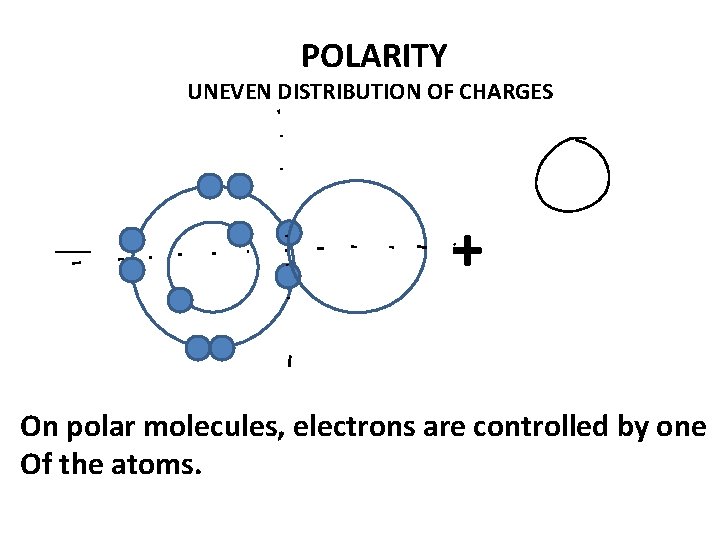
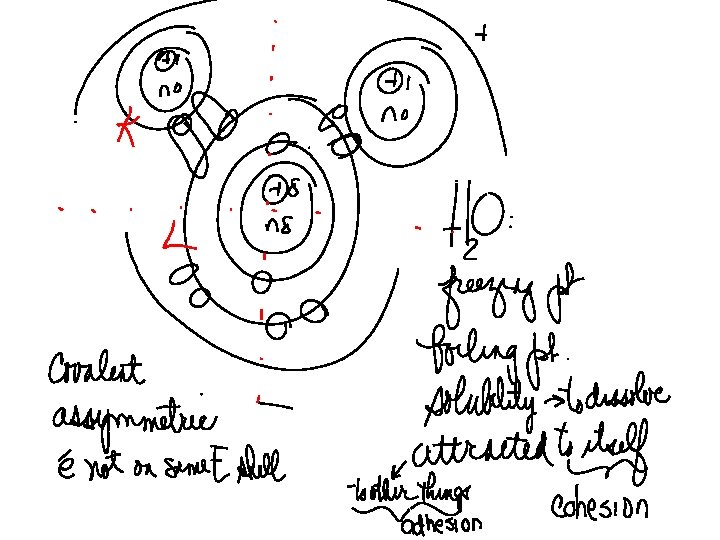
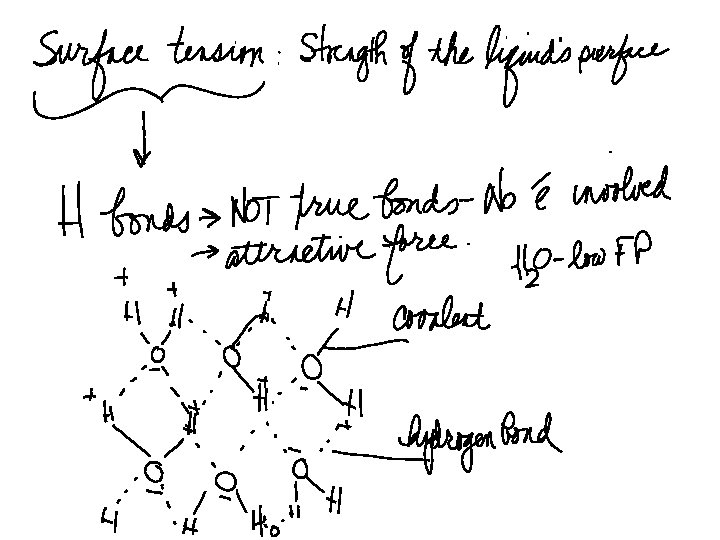
POLARITY UNEVEN DISTRIBUTION OF CHARGES __ + On polar molecules, electrons are controlled by one Of the atoms.


Video we viewed in class is How polarity makes water behave strangely - Christina Kleinberg On youtube.
- Slides: 14