Covalent Bonding Review Reactivity Stability Chemical bond Lewis

Covalent Bonding

Review § Reactivity § Stability § Chemical bond § Lewis dot symbol
Covalent bond § Bond in which two or more valence electrons are shared by two atoms § Occurs with elements close to each other on the periodic table § Between a nonmetal and a nonmetal § Ex: H 2 O, NH 3 (ammonia), CH 4 (methane)
3 Bond Types § Single Covalent Bond § Double Covalent Bond § Triple Covalent Bond
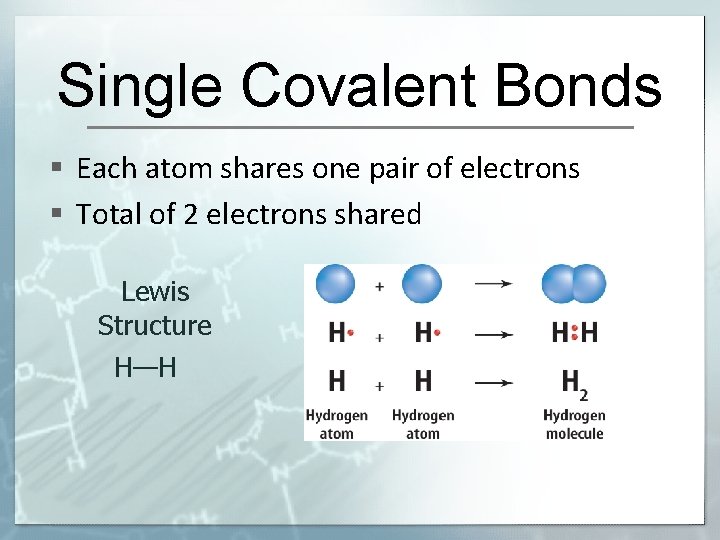
Single Covalent Bonds § Each atom shares one pair of electrons § Total of 2 electrons shared Lewis Structure H—H
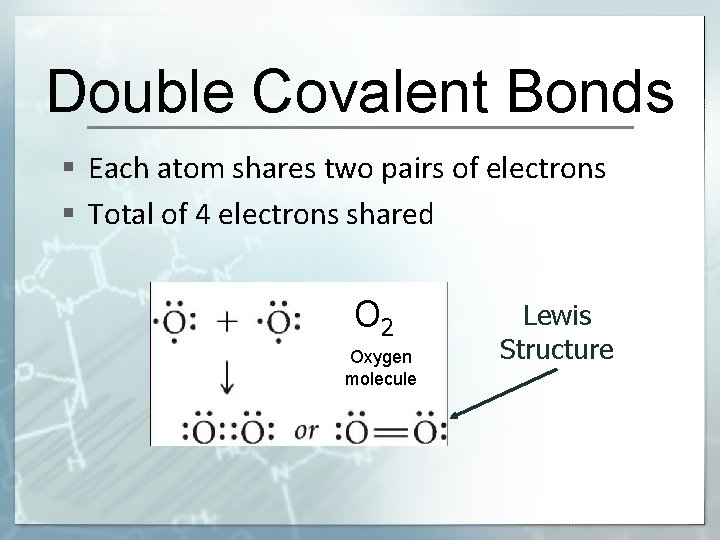
Double Covalent Bonds § Each atom shares two pairs of electrons § Total of 4 electrons shared O 2 Oxygen molecule Lewis Structure
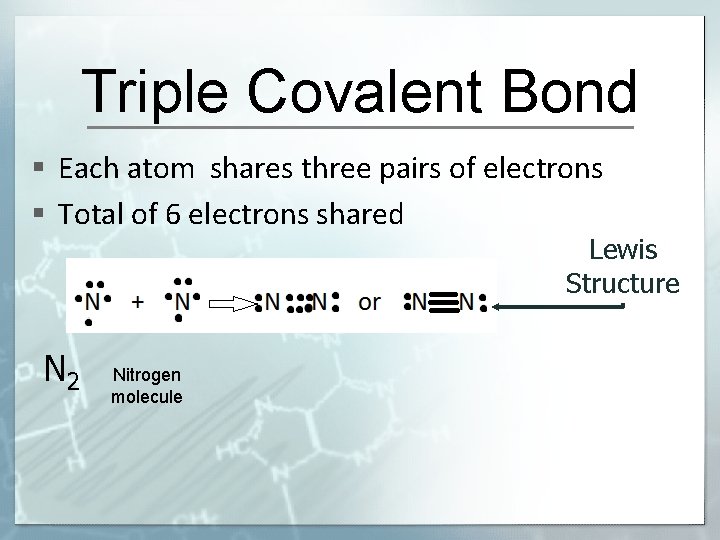
Triple Covalent Bond § Each atom shares three pairs of electrons § Total of 6 electrons shared Lewis Structure N 2 Nitrogen molecule
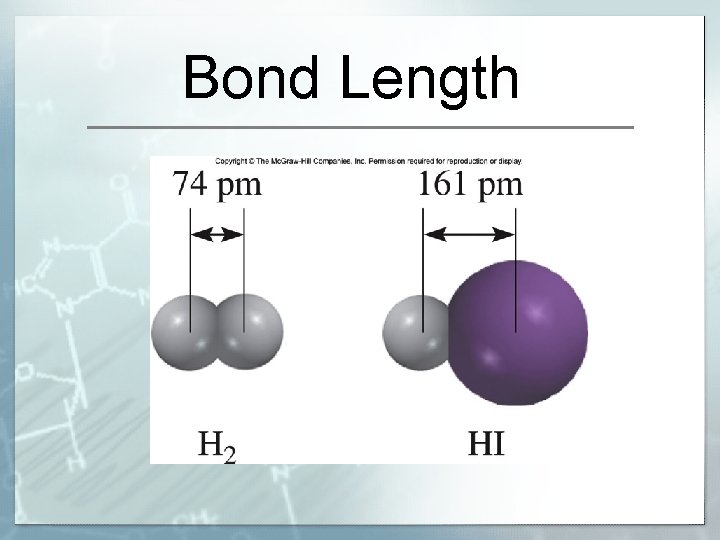
Bond Length
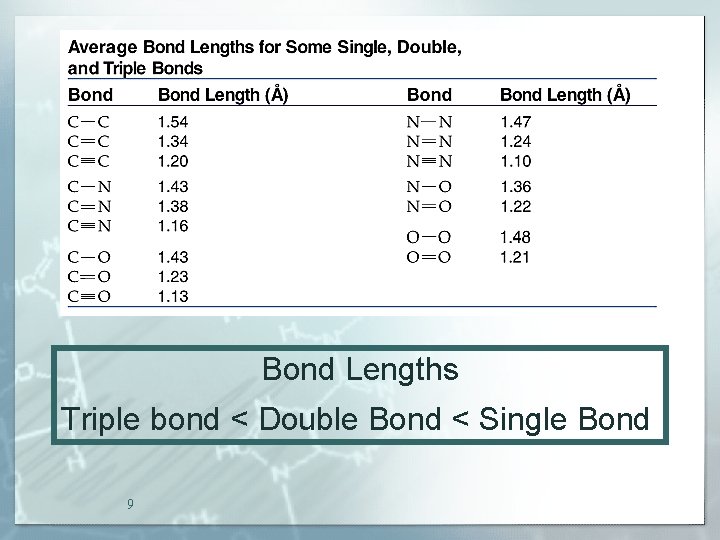
Bond Lengths Triple bond < Double Bond < Single Bond 9

Bond Strengths Triple bond > Double Bond > Single Bond
Electronegativity
Electronegativity Ability of an atom to attract towards itself electrons in a chemical bond
*video
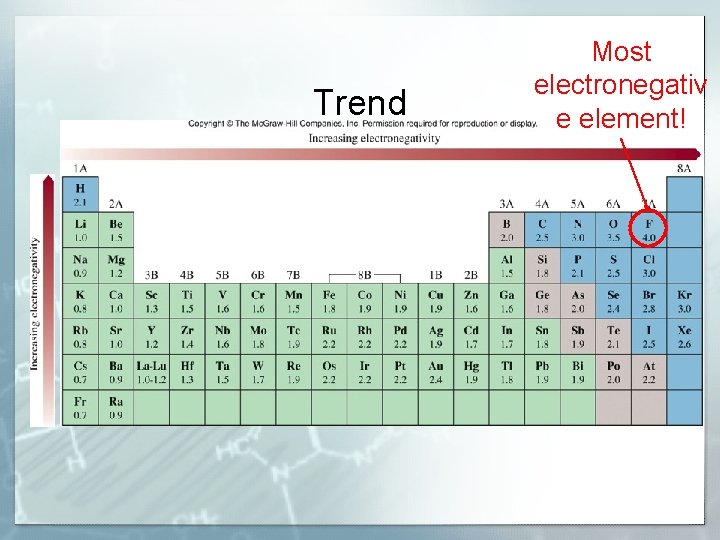
Trend Most electronegativ e element!
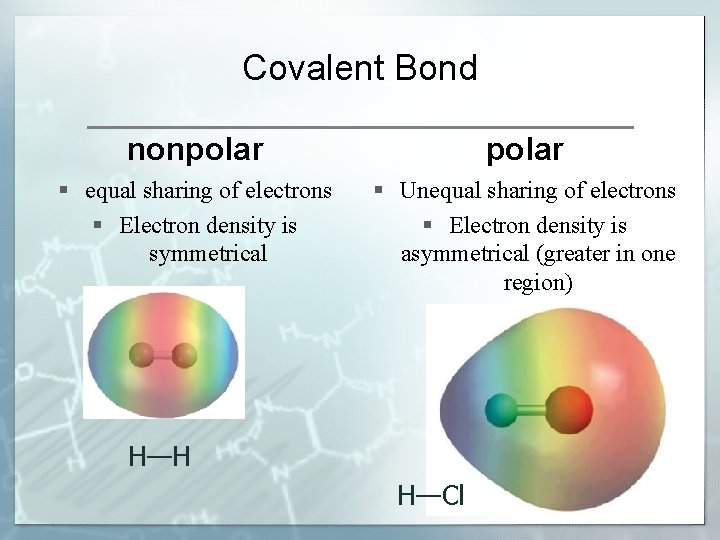
Covalent Bond nonpolar § equal sharing of electrons § Electron density is symmetrical § Unequal sharing of electrons § Electron density is asymmetrical (greater in one region) H—H H—Cl
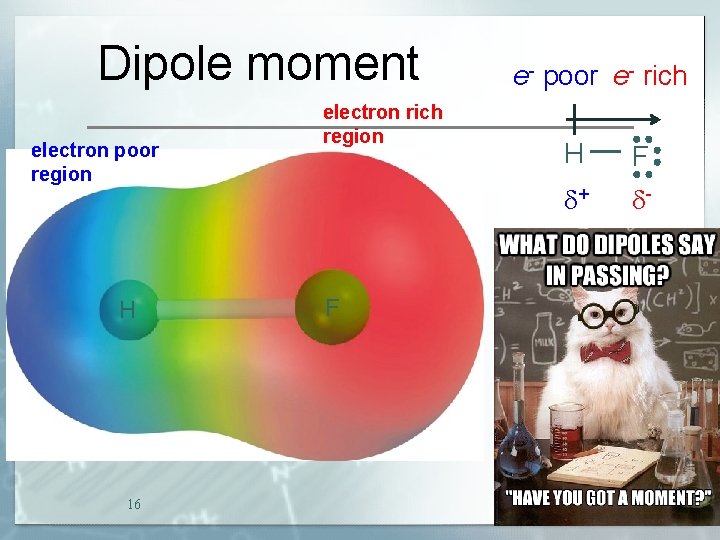
Dipole moment electron poor region H 16 electron rich region F e- poor e- rich H F d+ d-
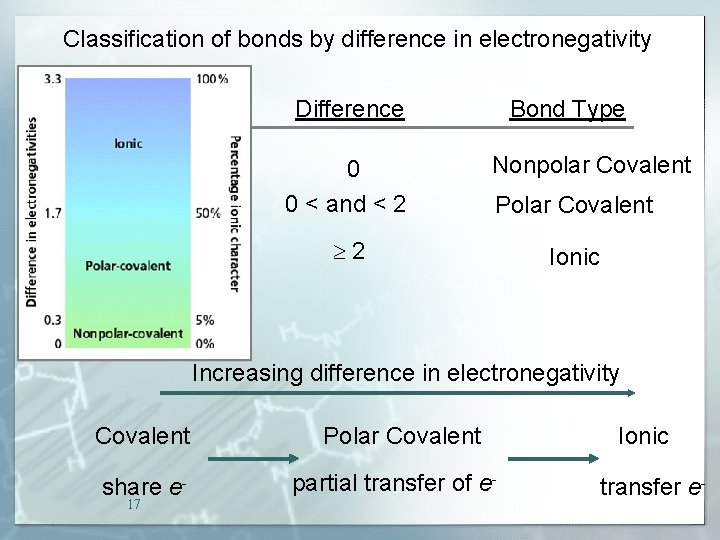
Classification of bonds by difference in electronegativity Difference 0 0 < and < 2 Bond Type Nonpolar Covalent Polar Covalent 2 Ionic Increasing difference in electronegativity Covalent share e 17 Polar Covalent partial transfer of e- Ionic transfer e-
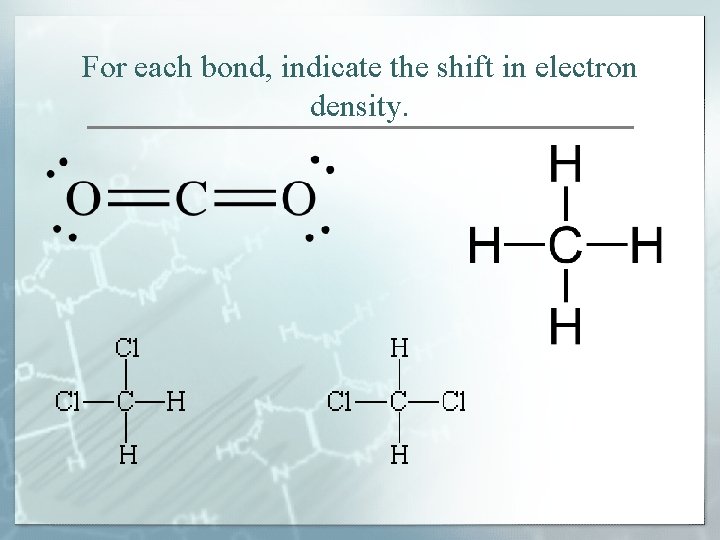
For each bond, indicate the shift in electron density.
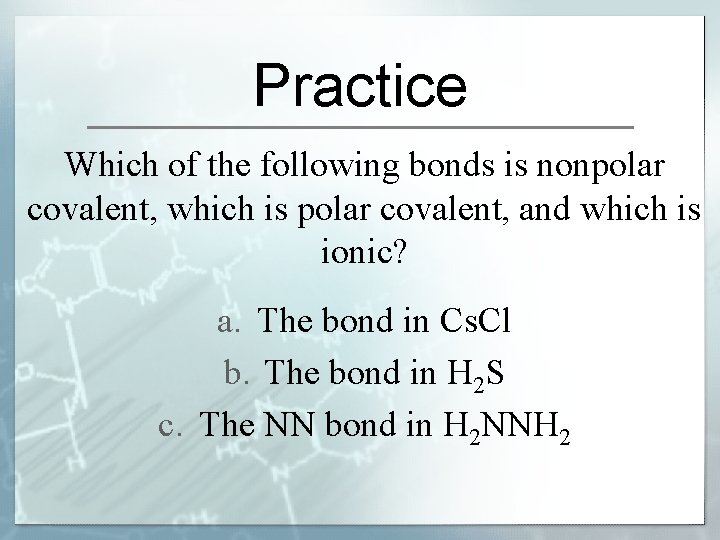
Practice Which of the following bonds is nonpolar covalent, which is polar covalent, and which is ionic? a. The bond in Cs. Cl b. The bond in H 2 S c. The NN bond in H 2 NNH 2
Substance Molecule Compound § Two or more atoms chemically bonded together § Two or more elements chemically bonded together
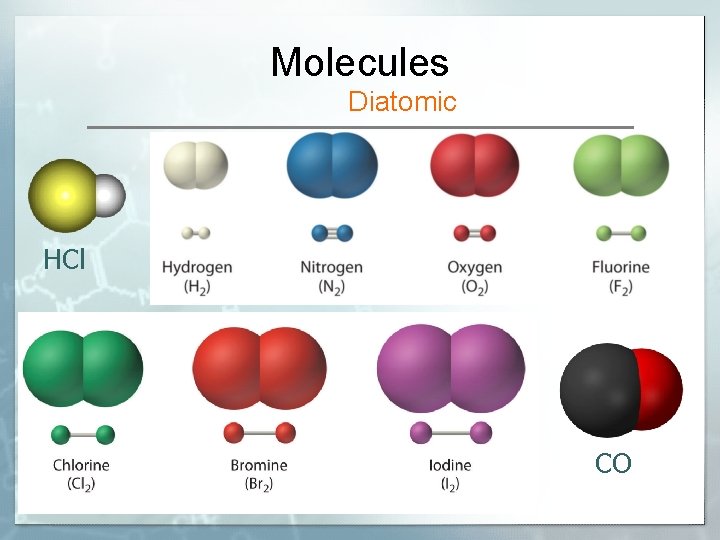
Molecules Diatomic HCl CO
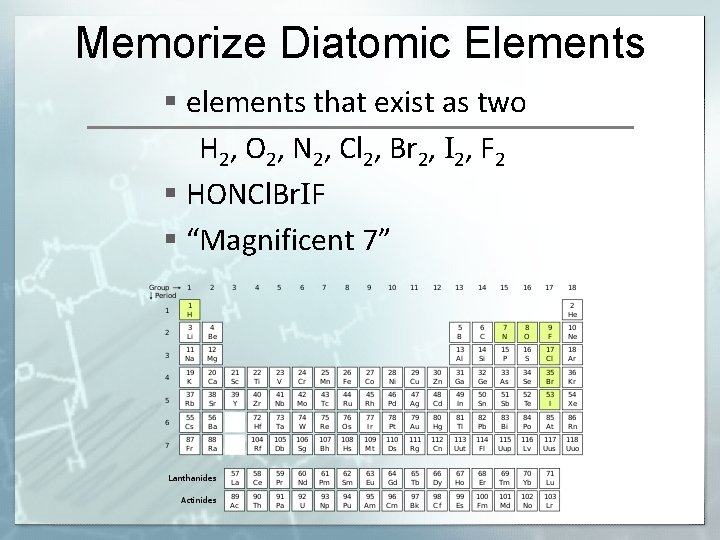
Memorize Diatomic Elements § elements that exist as two H 2, O 2, N 2, Cl 2, Br 2, I 2, F 2 § HONCl. Br. IF § “Magnificent 7”
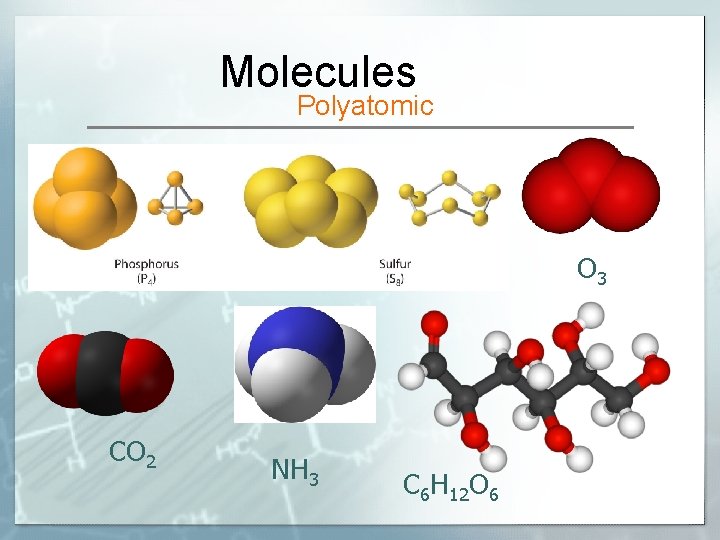
Molecules Polyatomic O 3 CO 2 NH 3 C 6 H 12 O 6
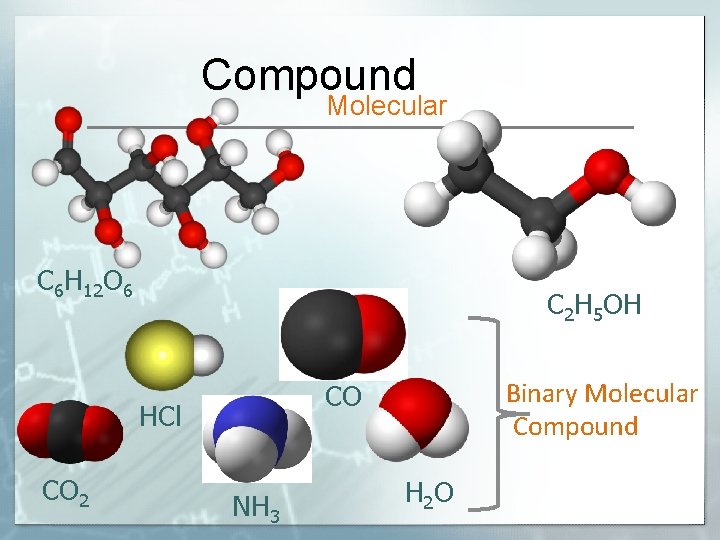
Compound Molecular C 6 H 12 O 6 C 2 H 5 OH HCl CO 2 Binary Molecular Compound CO NH 3 H 2 O
Intermolecular forces § Attractive forces between molecules § 3 types: § London dispersion § Dipole-dipole § Hydrogen bond
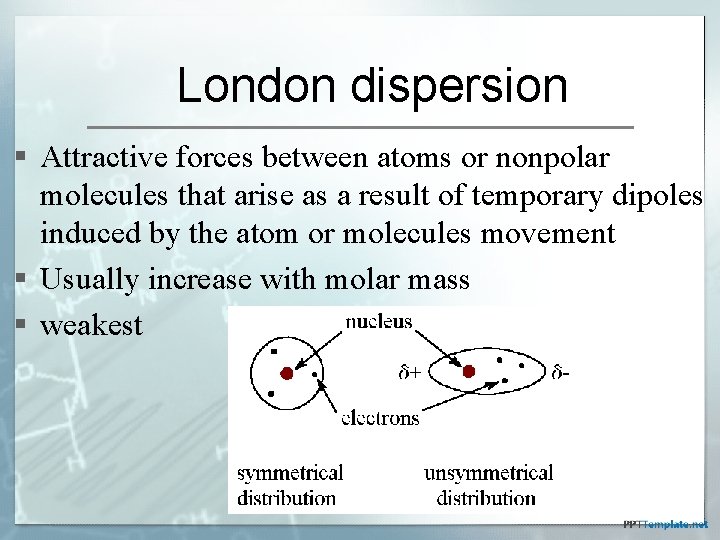
London dispersion § Attractive forces between atoms or nonpolar molecules that arise as a result of temporary dipoles induced by the atom or molecules movement § Usually increase with molar mass § weakest
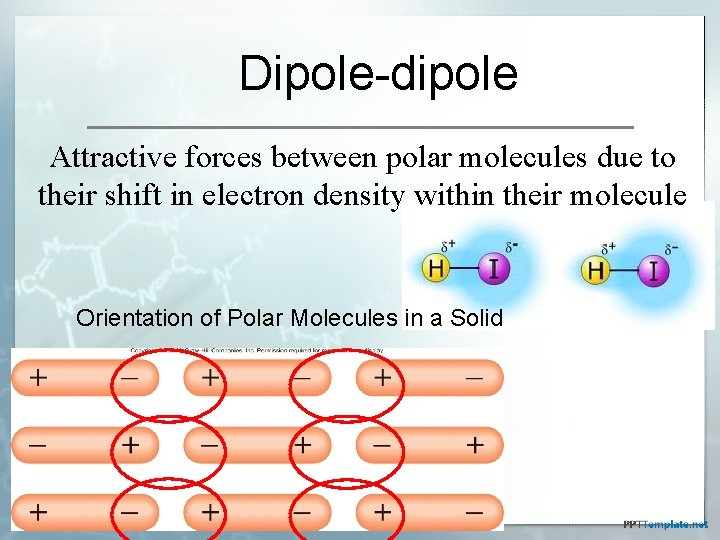
Dipole-dipole Attractive forces between polar molecules due to their shift in electron density within their molecule Orientation of Polar Molecules in a Solid
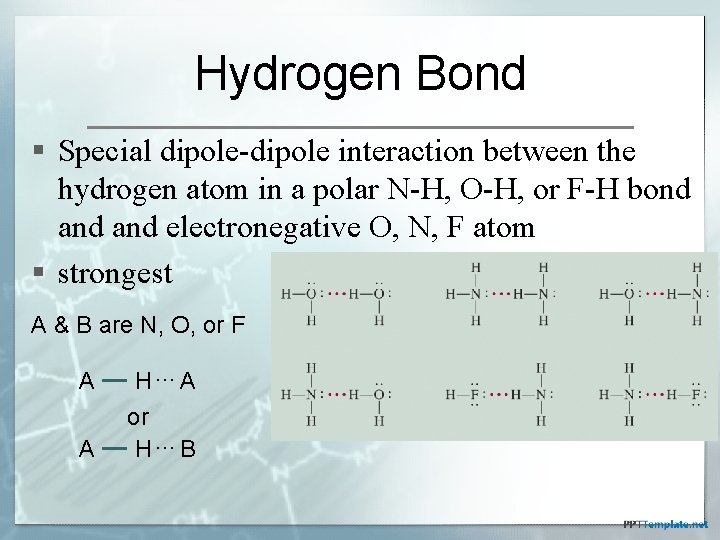
Hydrogen Bond § Special dipole-dipole interaction between the hydrogen atom in a polar N-H, O-H, or F-H bond and electronegative O, N, F atom § strongest A & B are N, O, or F A H…A A or H…B
Total Attraction § Hydrogen bond pre mi § Dipole-dipole § Dispersion V T t n re e f f i d s e e g k i a k *L c a p l e n n a ch § Dipole-dipole § Dispersion um ba sic
Molecular Compound Properties § Tend to be soft solids, liquids, or gases at room temperature § Low melting and boiling points § Poor conductors of heat and electricity § Non-electrolytes
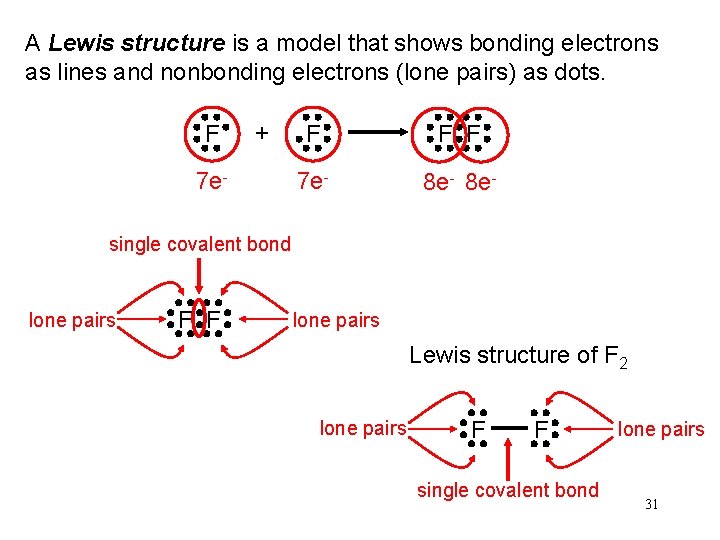
A Lewis structure is a model that shows bonding electrons as lines and nonbonding electrons (lone pairs) as dots. F + 7 e- F F F 7 e- 8 e- single covalent bond lone pairs F F lone pairs Lewis structure of F 2 lone pairs F F single covalent bond lone pairs 31
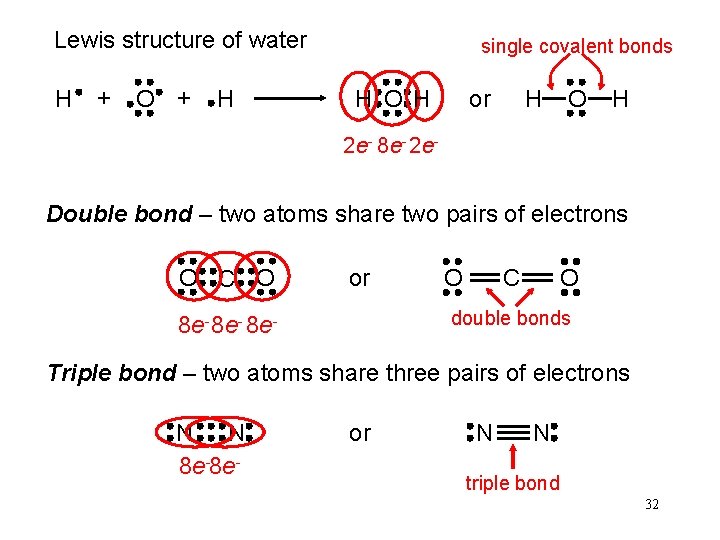
Lewis structure of water H + O + H single covalent bonds H O H H or O H 2 e- 8 e- 2 e- Double bond – two atoms share two pairs of electrons O C O or O O C double bonds 8 e- 8 e- Triple bond – two atoms share three pairs of electrons N N 8 e-8 e- or N N triple bond 32
- Slides: 32