Covalent Bonding Orbitals The Central Themes of VB

Covalent Bonding: Orbitals

The Central Themes of VB Theory Basic Principle • A covalent bond forms when the orbitals of two atoms overlap and are occupied by a pair of electrons that have the highest probability of being located between the nuclei. Themes • These overlapping orbitals can have up to two electrons that must have opposite spins (Pauli principle). • The valence orbitals in a molecule are different from those in isolated atoms. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 2
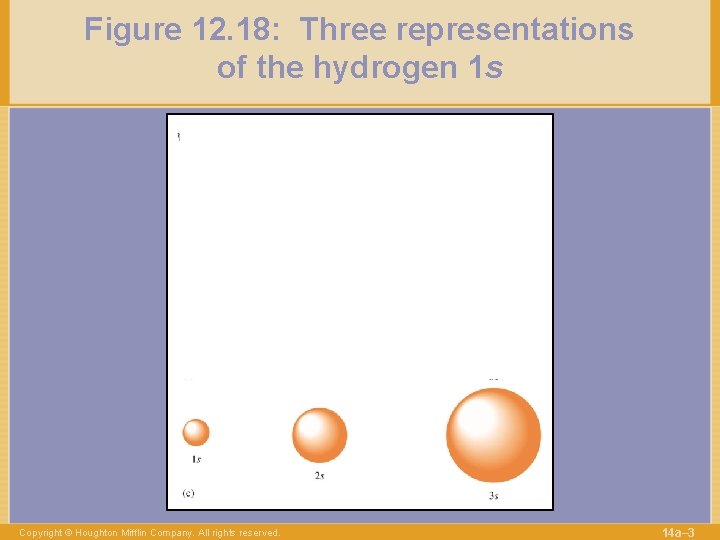
Figure 12. 18: Three representations of the hydrogen 1 s Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 3
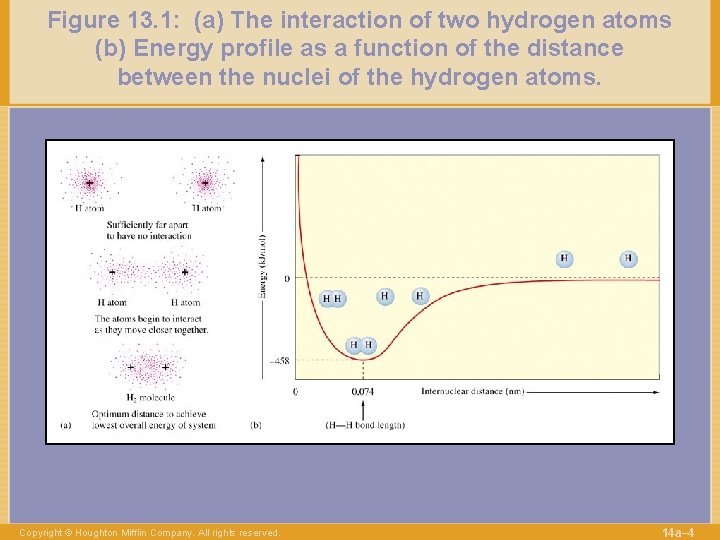
Figure 13. 1: (a) The interaction of two hydrogen atoms (b) Energy profile as a function of the distance between the nuclei of the hydrogen atoms. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 4
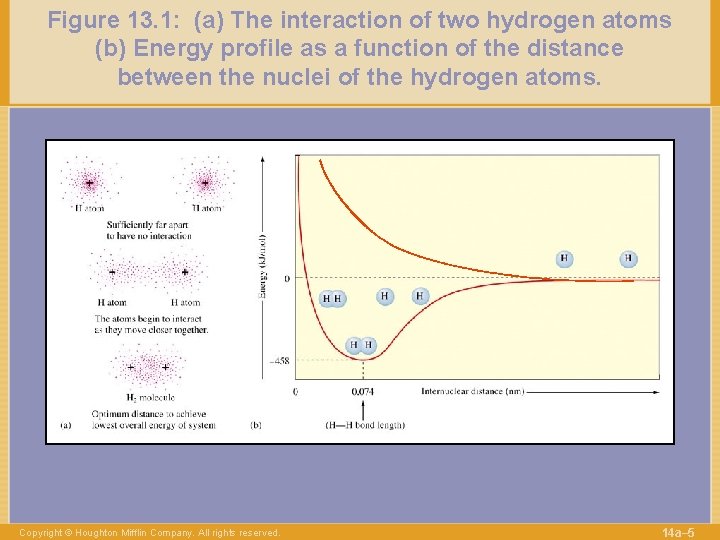
Figure 13. 1: (a) The interaction of two hydrogen atoms (b) Energy profile as a function of the distance between the nuclei of the hydrogen atoms. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 5
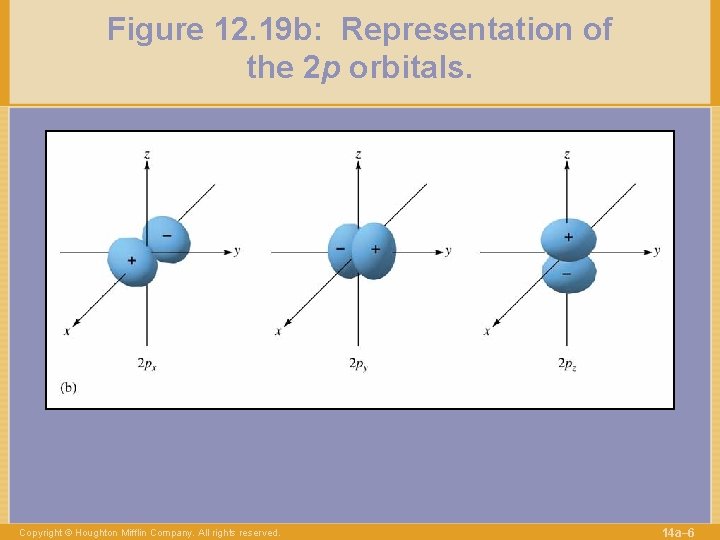
Figure 12. 19 b: Representation of the 2 p orbitals. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 6
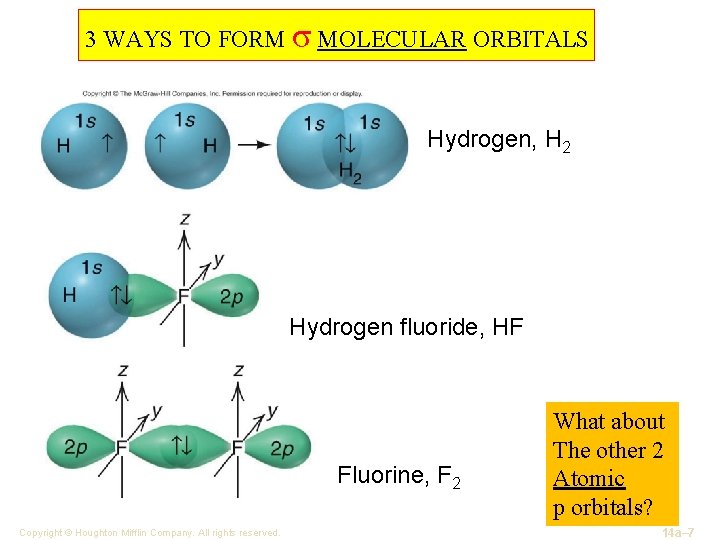
3 WAYS TO FORM σ MOLECULAR ORBITALS Hydrogen, H 2 Hydrogen fluoride, HF Fluorine, F 2 Copyright © Houghton Mifflin Company. All rights reserved. What about The other 2 Atomic p orbitals? 14 a– 7

Figure 14. 1: (a) Lewis structure of the methane molecule (b) the tetrahedral molecular geometry of the methane molecule. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 8
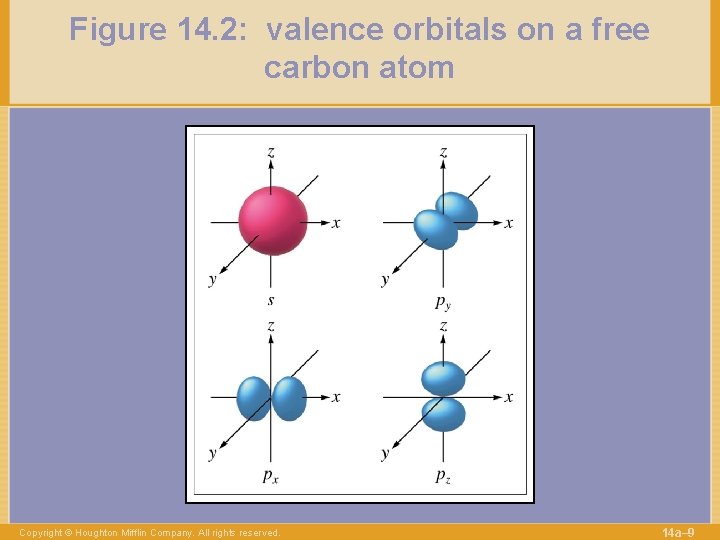
Figure 14. 2: valence orbitals on a free carbon atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 9
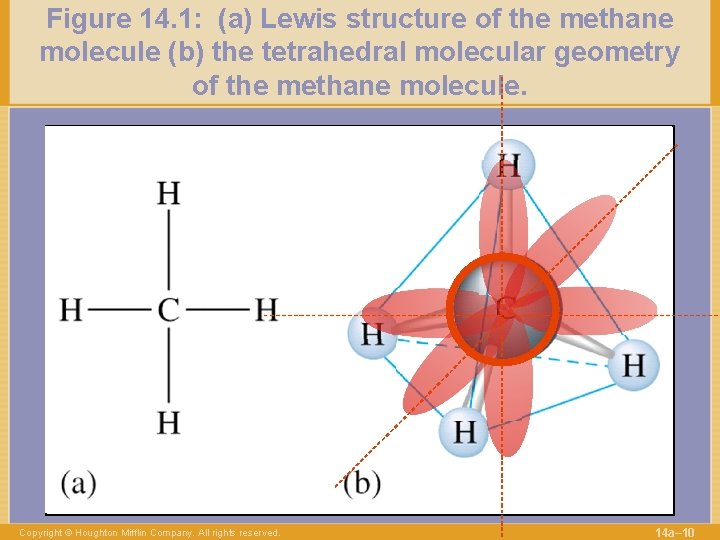
Figure 14. 1: (a) Lewis structure of the methane molecule (b) the tetrahedral molecular geometry of the methane molecule. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 10
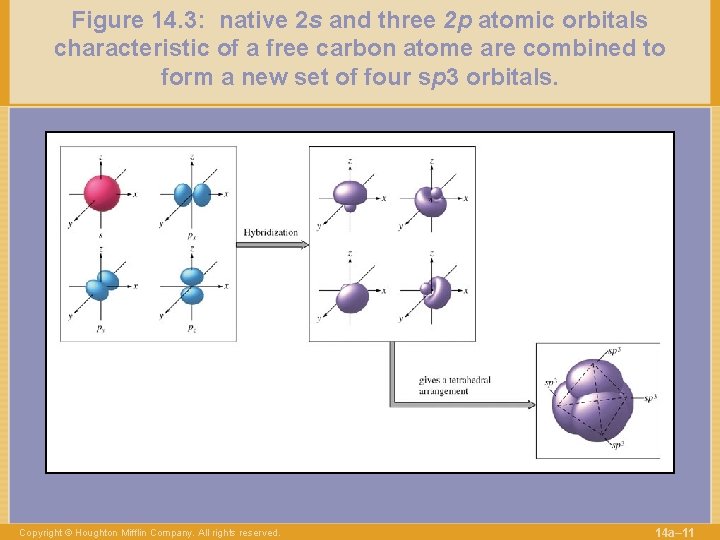
Figure 14. 3: native 2 s and three 2 p atomic orbitals characteristic of a free carbon atome are combined to form a new set of four sp 3 orbitals. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 11
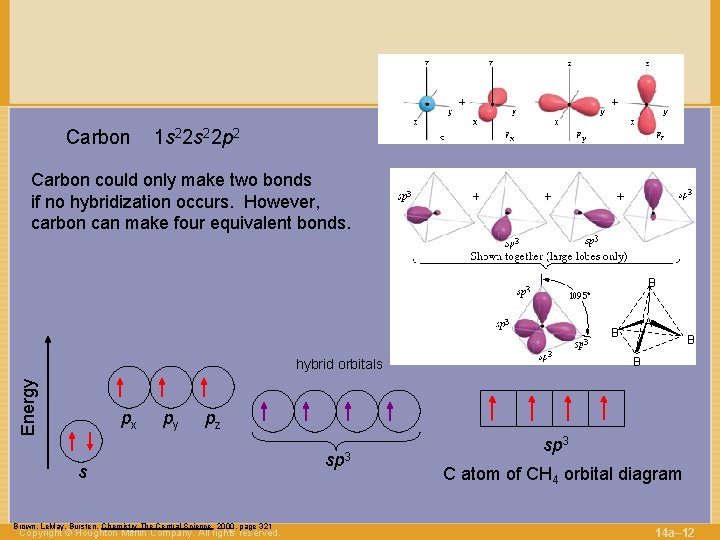
Carbon 1 s 22 p 2 Carbon could only make two bonds if no hybridization occurs. However, carbon can make four equivalent bonds. B A B B Energy hybrid orbitals px py B pz s Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 321 Copyright © Houghton Mifflin Company. All rights reserved. sp 3 C atom of CH 4 orbital diagram 14 a– 12
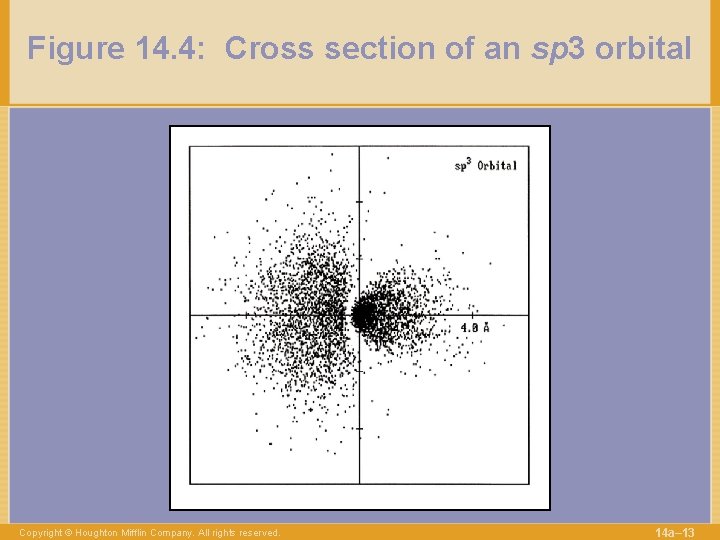
Figure 14. 4: Cross section of an sp 3 orbital Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 13
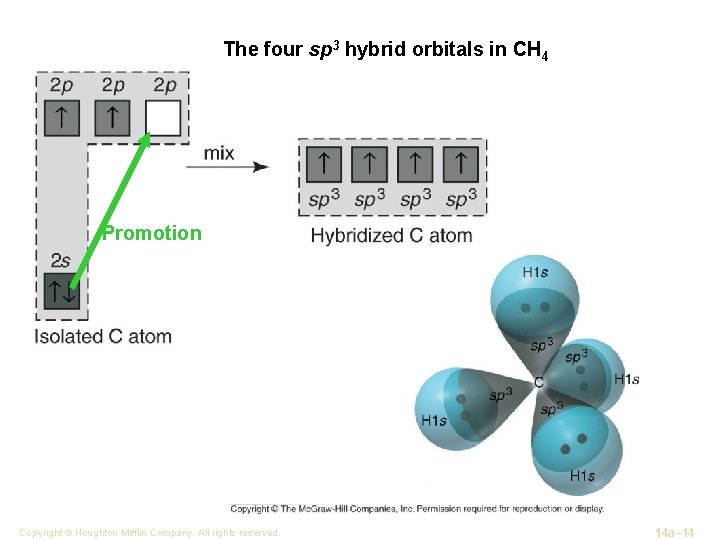
The four sp 3 hybrid orbitals in CH 4 Promotion Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 14
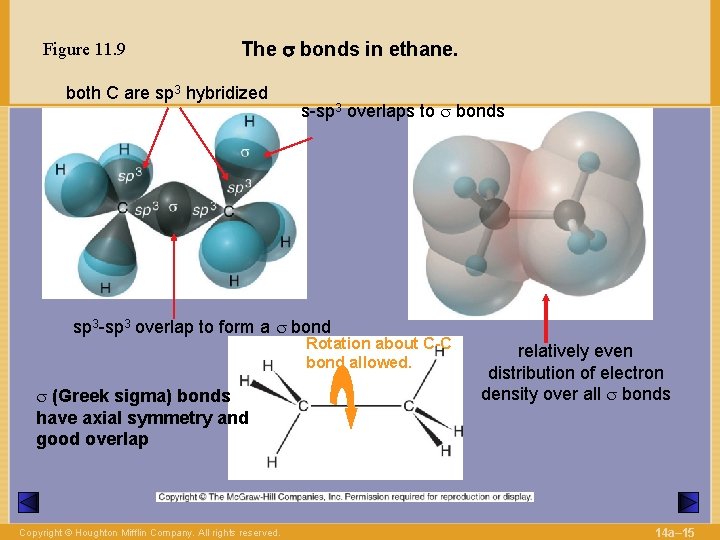
Figure 11. 9 The s bonds in ethane. both C are sp 3 hybridized s-sp 3 overlaps to s bonds sp 3 -sp 3 overlap to form a s bond Rotation about C-C bond allowed. s (Greek sigma) bonds have axial symmetry and good overlap Copyright © Houghton Mifflin Company. All rights reserved. relatively even distribution of electron density over all s bonds 14 a– 15
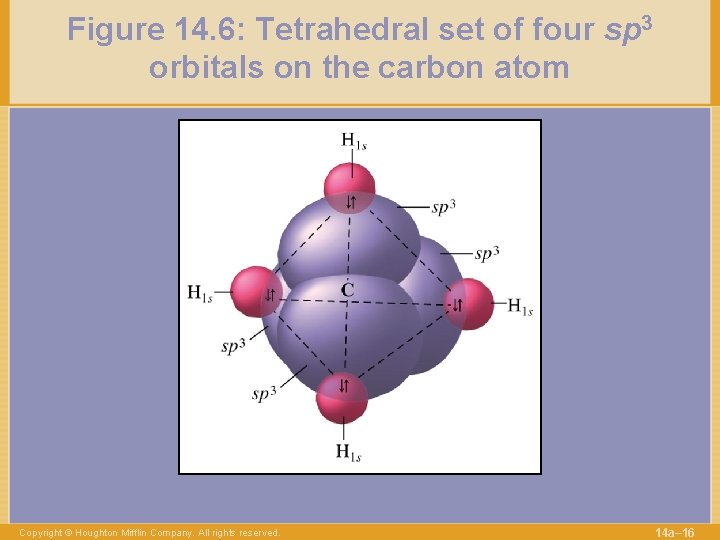
Figure 14. 6: Tetrahedral set of four sp 3 orbitals on the carbon atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 16
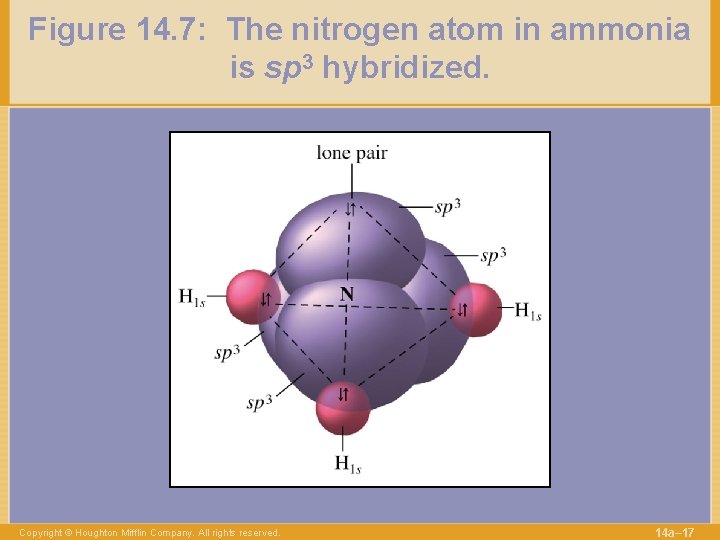
Figure 14. 7: The nitrogen atom in ammonia is sp 3 hybridized. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 17
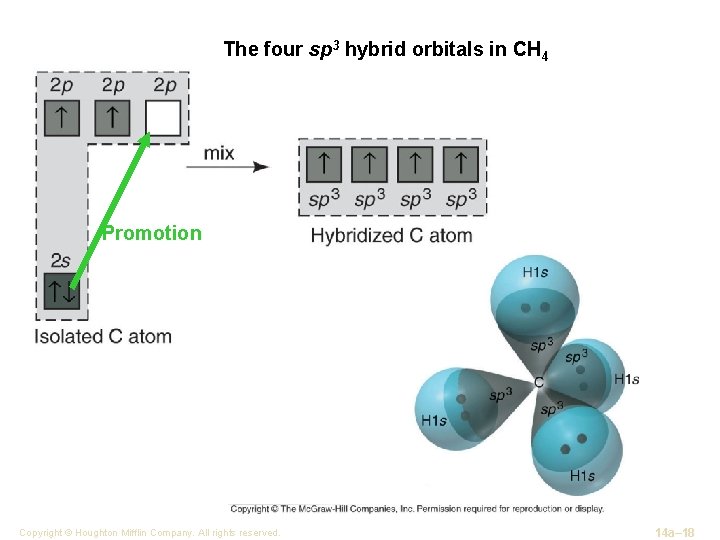
The four sp 3 hybrid orbitals in CH 4 Promotion Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 18
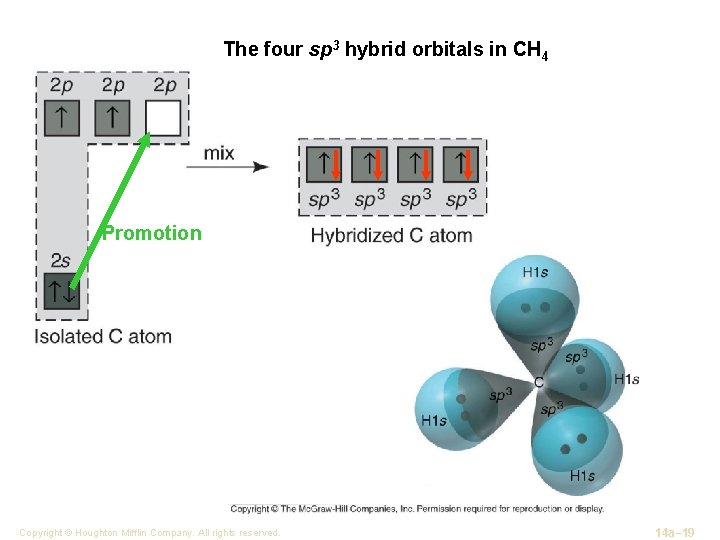
The four sp 3 hybrid orbitals in CH 4 Promotion Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 19
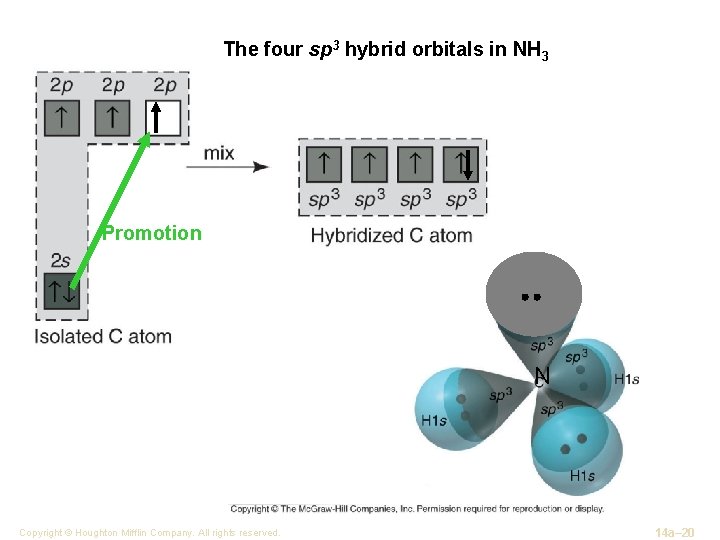
The four sp 3 hybrid orbitals in NH 3 Promotion N Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 20
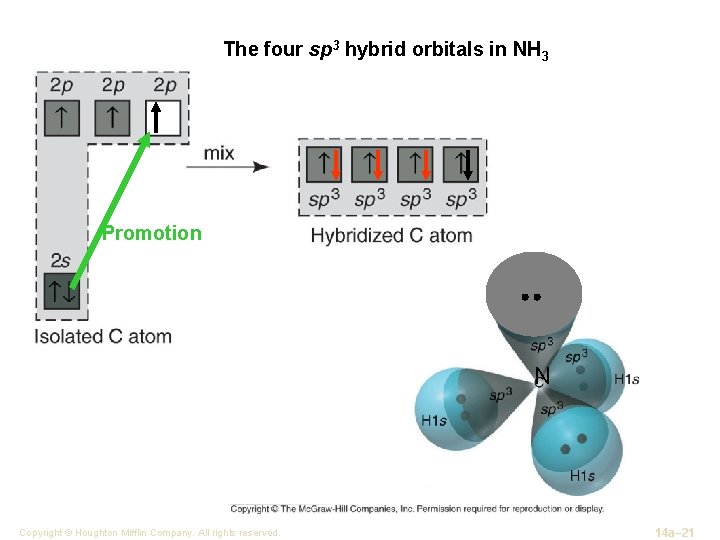
The four sp 3 hybrid orbitals in NH 3 Promotion N Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 21
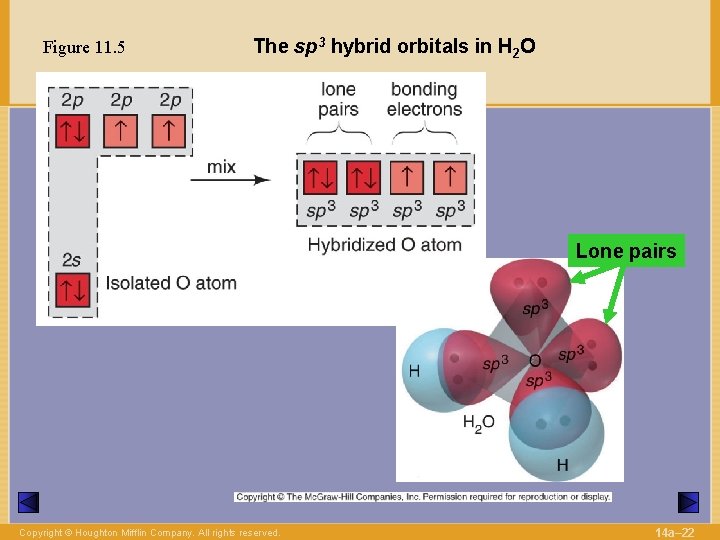
Figure 11. 5 The sp 3 hybrid orbitals in H 2 O Lone pairs Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 22
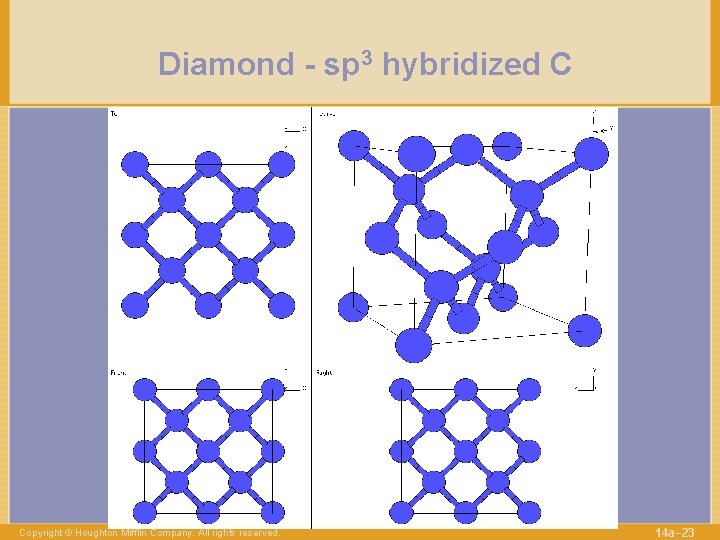
Diamond - sp 3 hybridized C Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 23
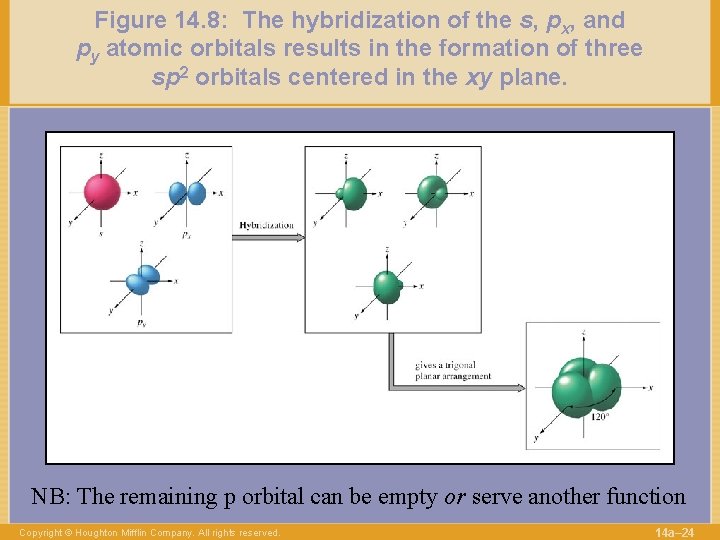
Figure 14. 8: The hybridization of the s, px, and py atomic orbitals results in the formation of three sp 2 orbitals centered in the xy plane. NB: The remaining p orbital can be empty or serve another function Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 24
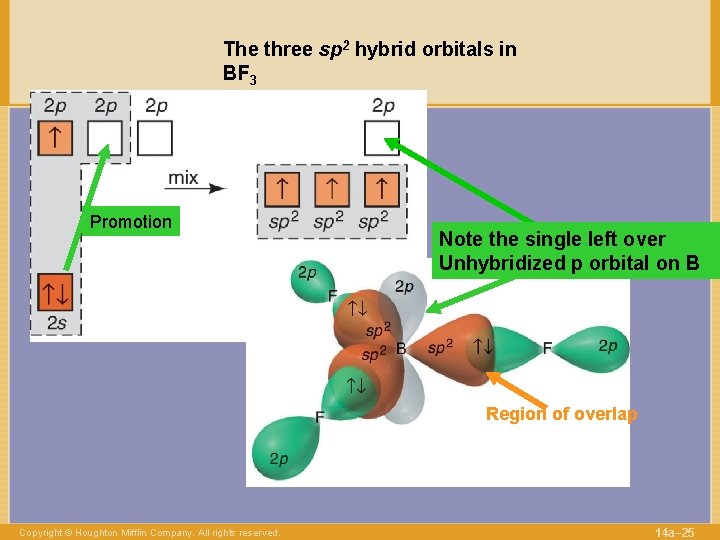
The three sp 2 hybrid orbitals in BF 3 Promotion Note the single left over Unhybridized p orbital on B Region of overlap Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 25
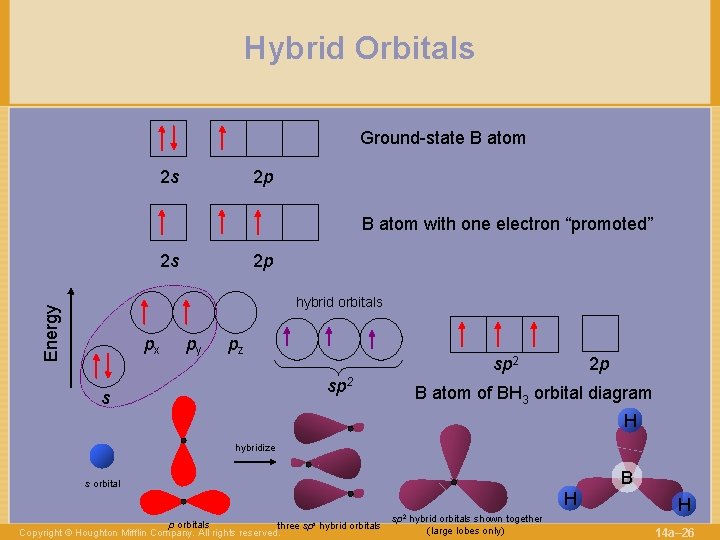
Hybrid Orbitals Ground-state B atom 2 s 2 p B atom with one electron “promoted” 2 s 2 p Energy hybrid orbitals px py pz sp 2 s 2 p B atom of BH 3 orbital diagram H hybridize B s orbital p orbitals three sps hybrid orbitals Copyright © Houghton Mifflin Company. All rights reserved. H sp 2 hybrid orbitals shown together (large lobes only) H 14 a– 26
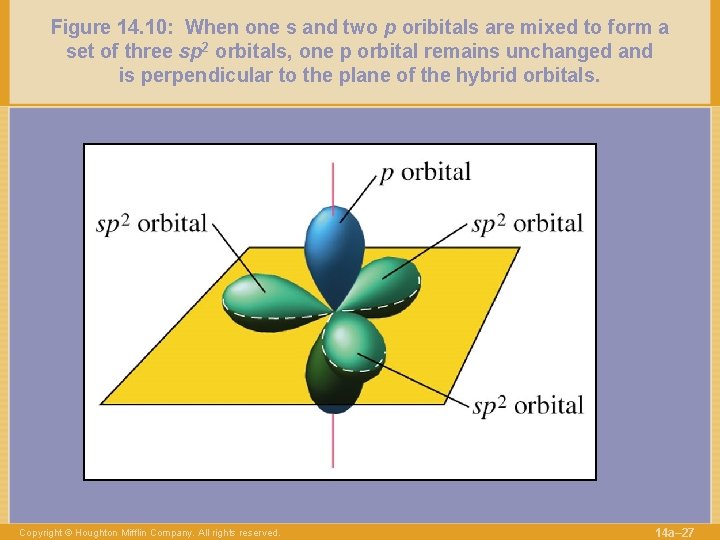
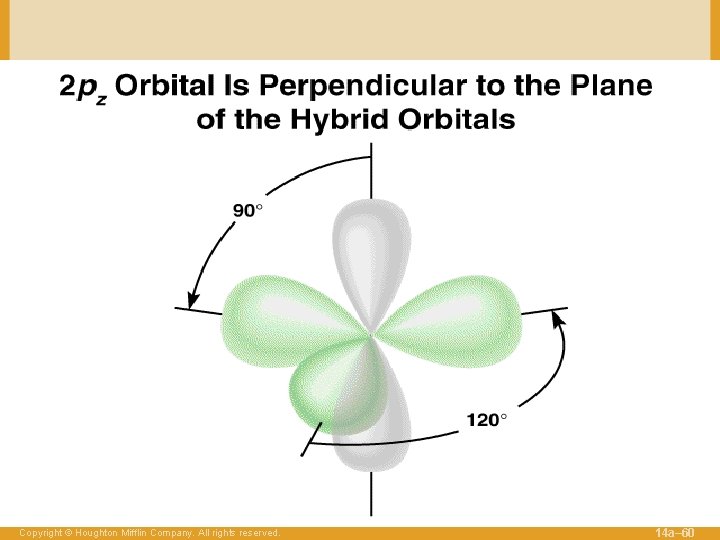
Figure 14. 10: When one s and two p oribitals are mixed to form a set of three sp 2 orbitals, one p orbital remains unchanged and is perpendicular to the plane of the hybrid orbitals. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 27
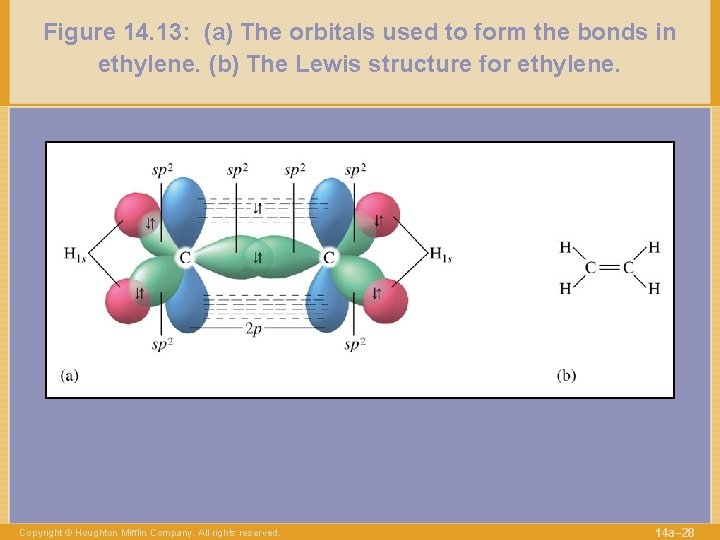
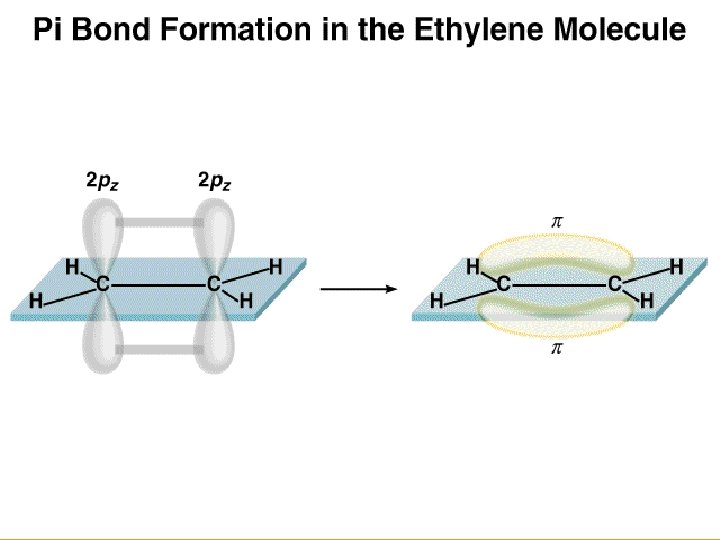
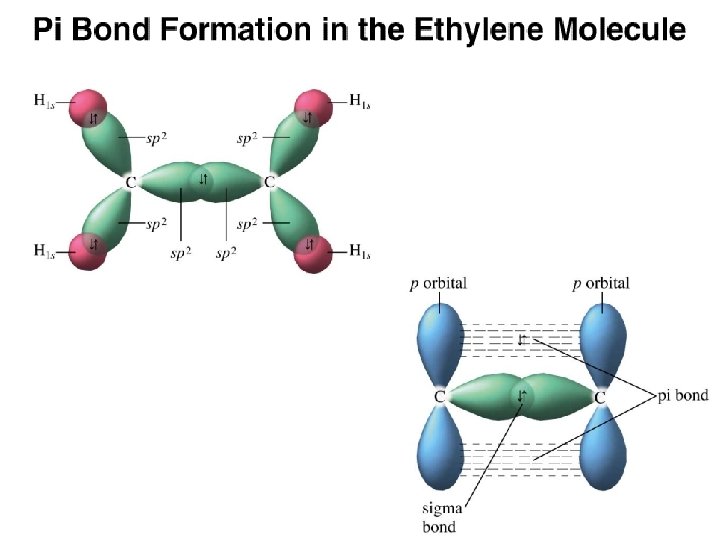
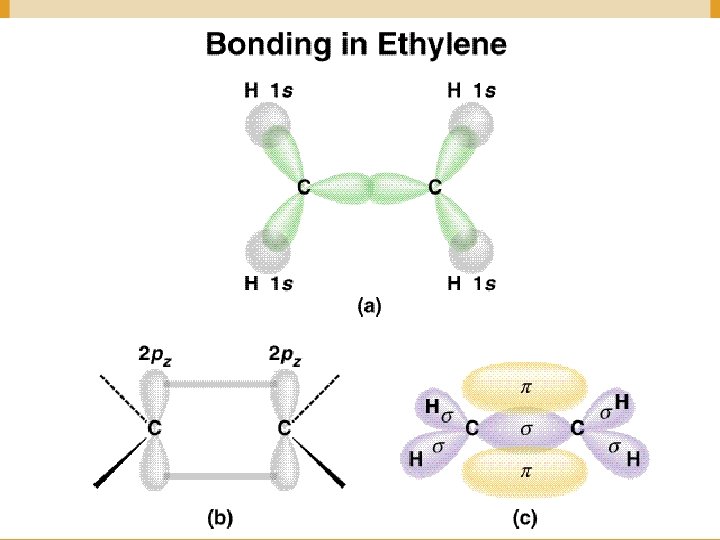
Figure 14. 13: (a) The orbitals used to form the bonds in ethylene. (b) The Lewis structure for ethylene. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 28

The plastics shown here were manufactured with ethylene. Source: Comstock - Mountainside, NJ Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 29
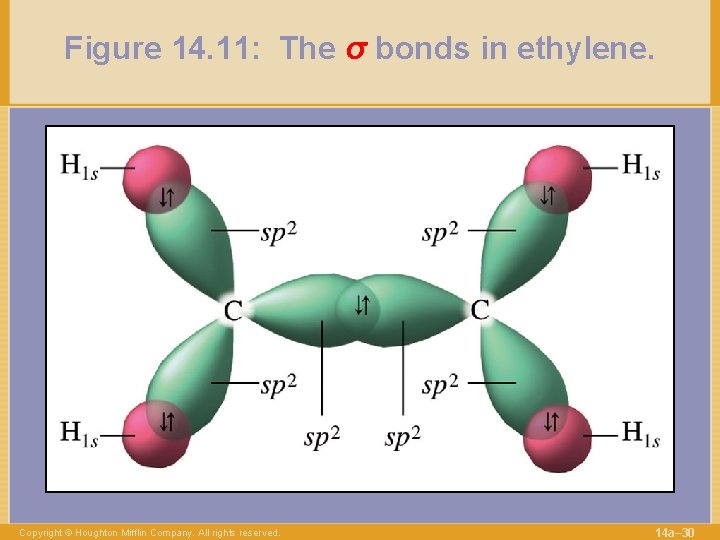
Figure 14. 11: The σ bonds in ethylene. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 30
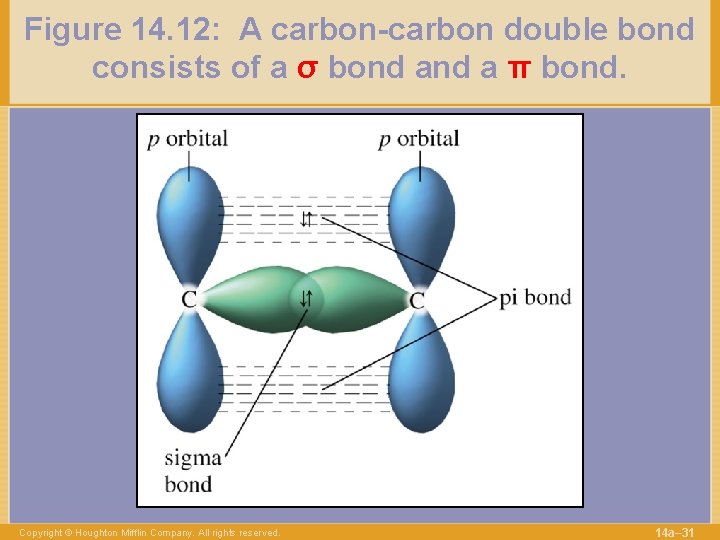
Figure 14. 12: A carbon-carbon double bond consists of a σ bond a π bond. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 31
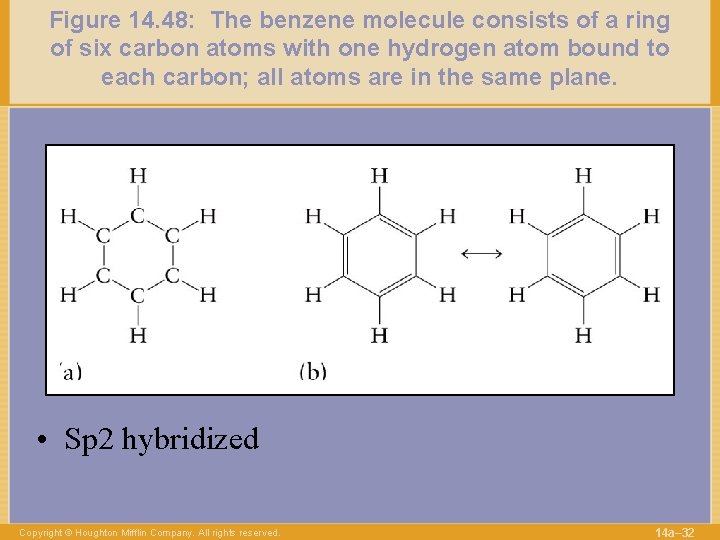
Figure 14. 48: The benzene molecule consists of a ring of six carbon atoms with one hydrogen atom bound to each carbon; all atoms are in the same plane. • Sp 2 hybridized Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 32
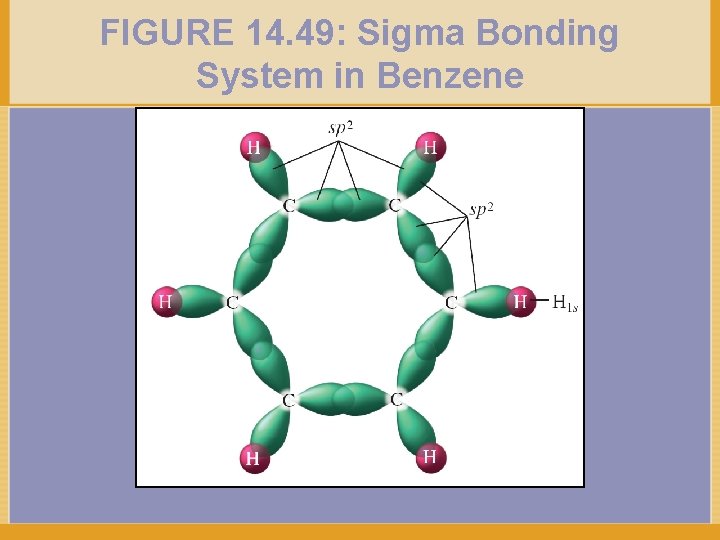
FIGURE 14. 49: Sigma Bonding System in Benzene
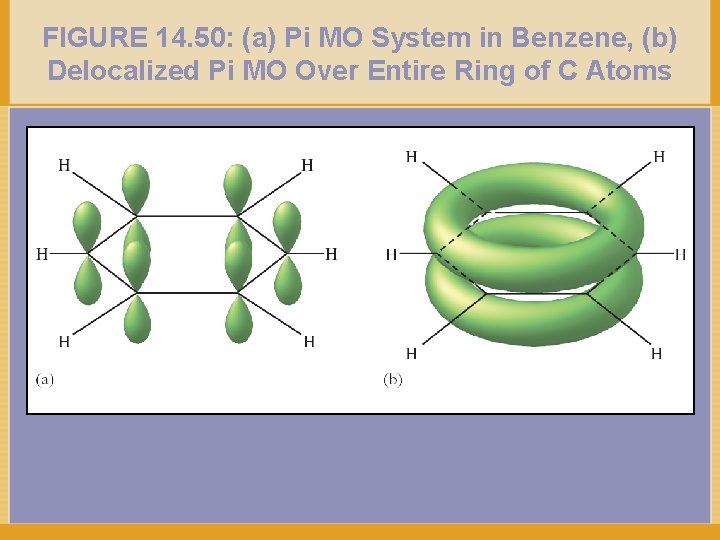
FIGURE 14. 50: (a) Pi MO System in Benzene, (b) Delocalized Pi MO Over Entire Ring of C Atoms
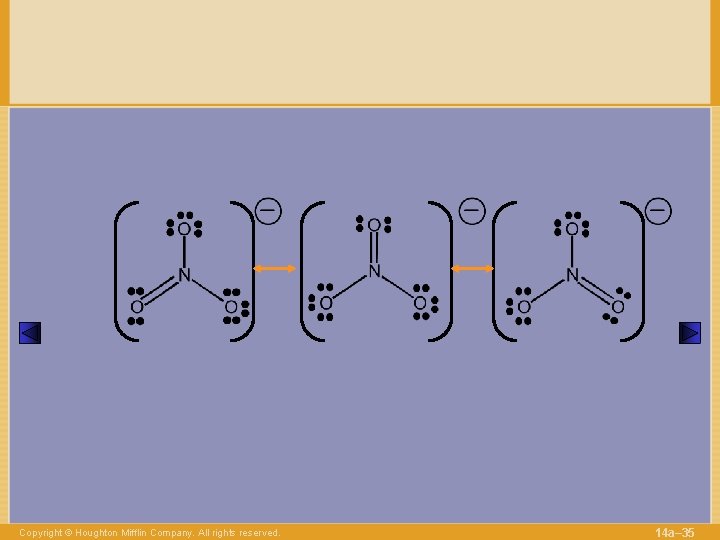
Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 35
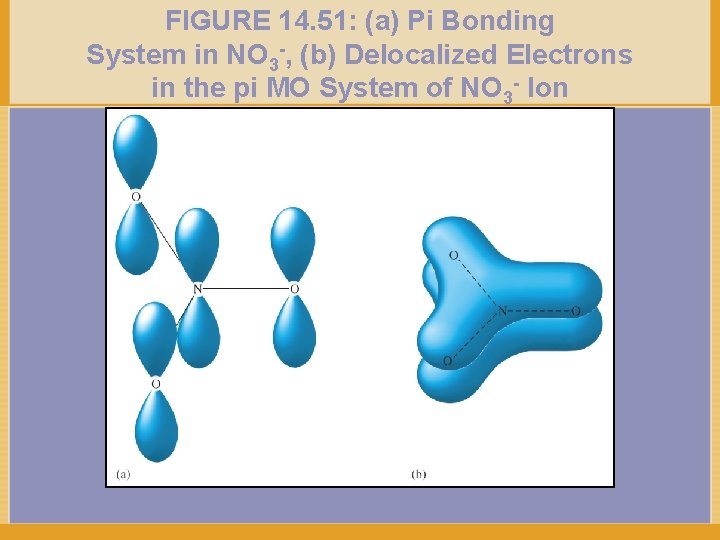
FIGURE 14. 51: (a) Pi Bonding System in NO 3 -, (b) Delocalized Electrons in the pi MO System of NO 3 - Ion
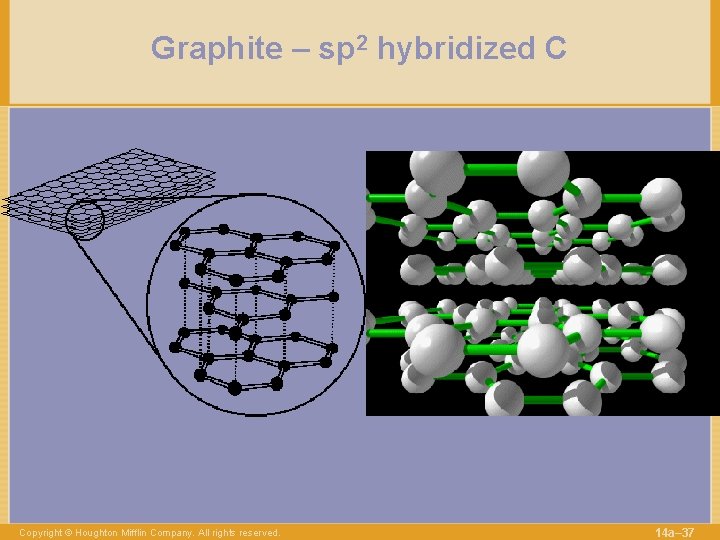
Graphite – sp 2 hybridized C Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 37
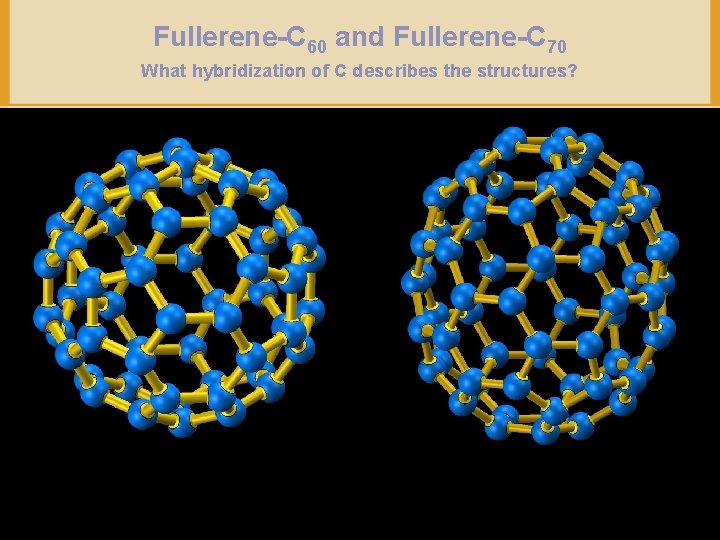
Fullerene-C 60 and Fullerene-C 70 What hybridization of C describes the structures? Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 38
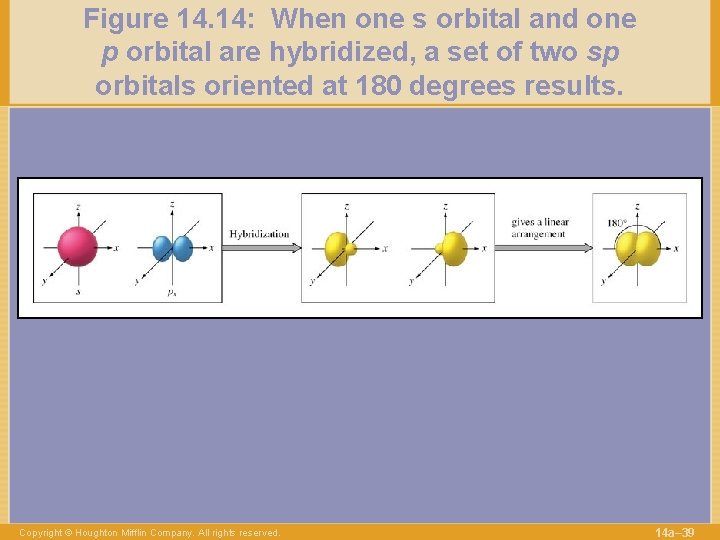
Figure 14. 14: When one s orbital and one p orbital are hybridized, a set of two sp orbitals oriented at 180 degrees results. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 39
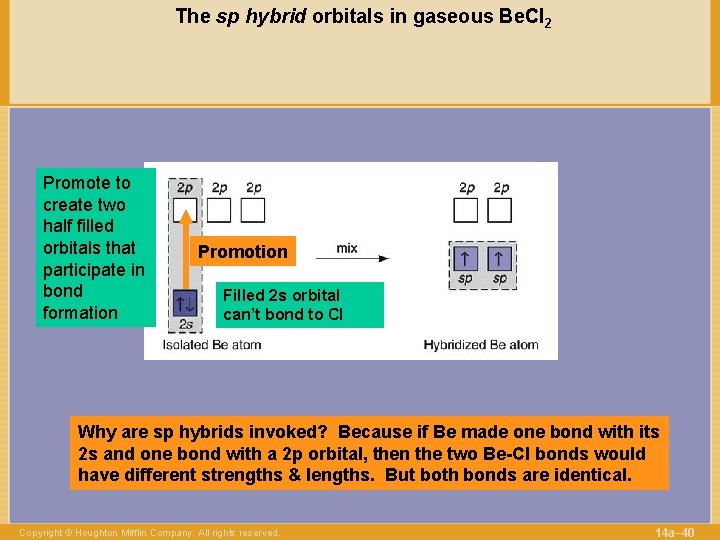
The sp hybrid orbitals in gaseous Be. Cl 2 Promote to create two half filled orbitals that participate in bond formation Promotion Filled 2 s orbital can’t bond to Cl Why are sp hybrids invoked? Because if Be made one bond with its 2 s and one bond with a 2 p orbital, then the two Be-Cl bonds would have different strengths & lengths. But both bonds are identical. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 40
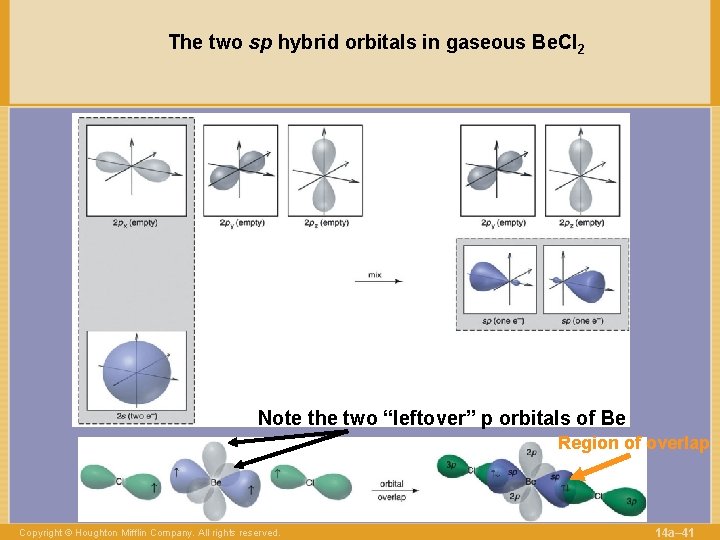
The two sp hybrid orbitals in gaseous Be. Cl 2 Note the two “leftover” p orbitals of Be Region of overlap Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 41
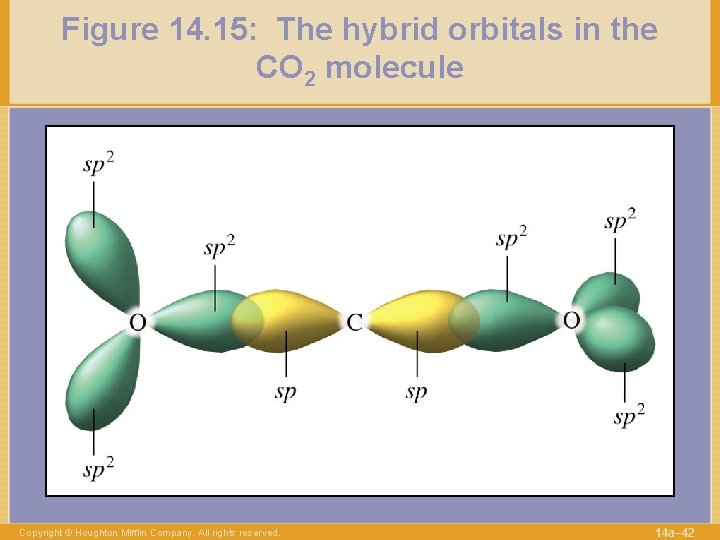
Figure 14. 15: The hybrid orbitals in the CO 2 molecule Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 42
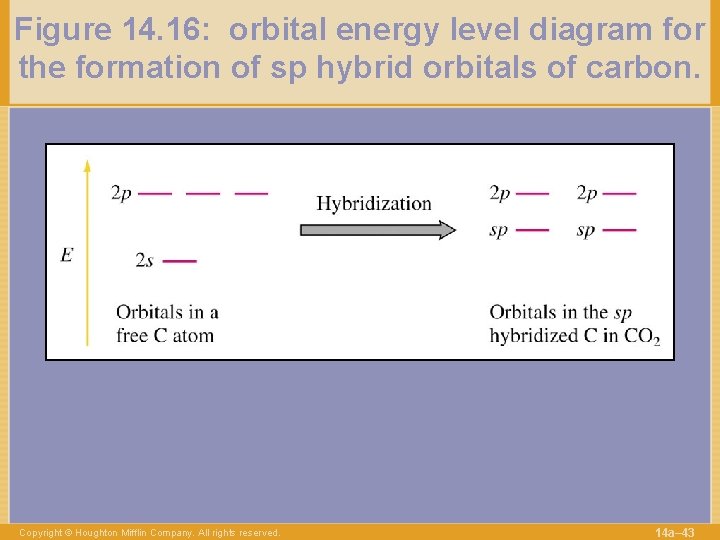
Figure 14. 16: orbital energy level diagram for the formation of sp hybrid orbitals of carbon. Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 43
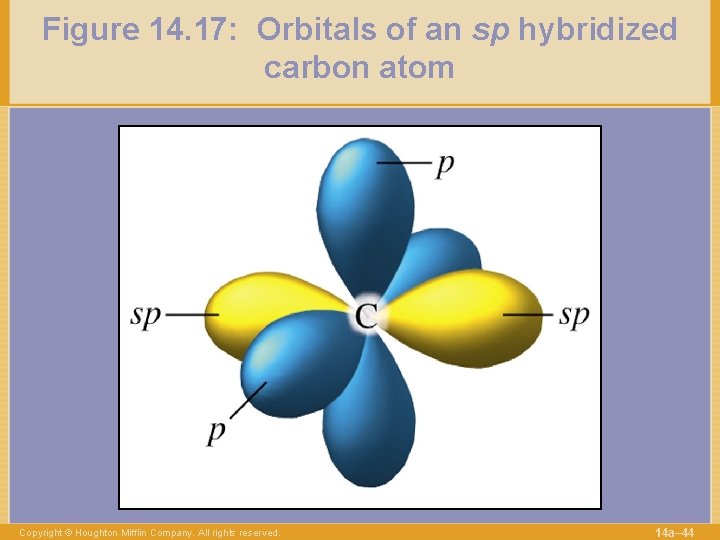
Figure 14. 17: Orbitals of an sp hybridized carbon atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 44
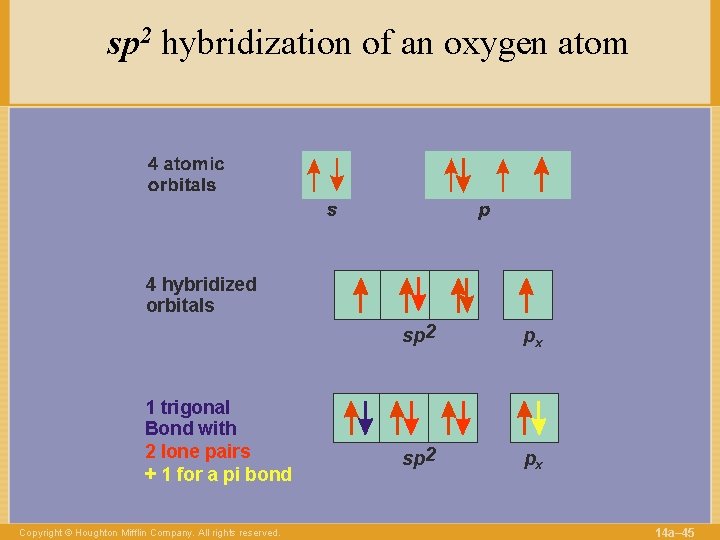
sp 2 hybridization of an oxygen atom 4 hybridized orbitals 1 trigonal Bond with 2 lone pairs + 1 for a pi bond Copyright © Houghton Mifflin Company. All rights reserved. sp 2 px 14 a– 45
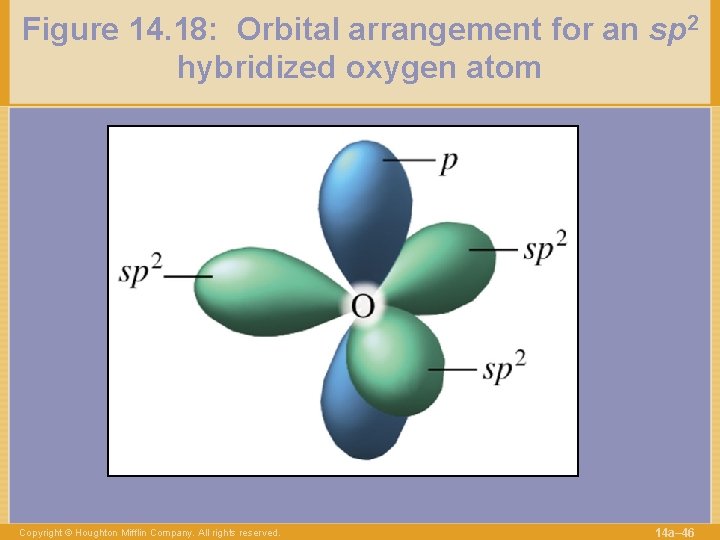
Figure 14. 18: Orbital arrangement for an sp 2 hybridized oxygen atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 46
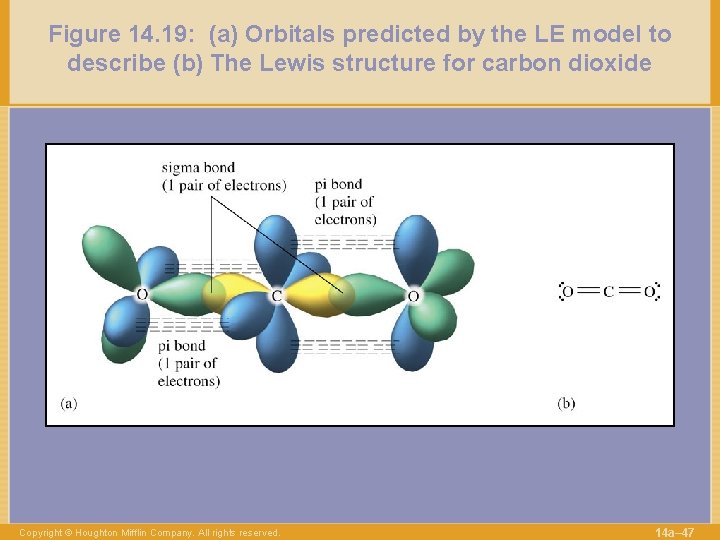
Figure 14. 19: (a) Orbitals predicted by the LE model to describe (b) The Lewis structure for carbon dioxide Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 47

Hybrid Orbitals Types of Hybrid Orbitals sp Shapes: linear # orbitals: 2 sp 2 triangular 3 Copyright © Houghton Mifflin Company. All rights reserved. sp 3 d 2 tetrahedral trig. bipyram. Octahedral 4 5 6 14 a– 48
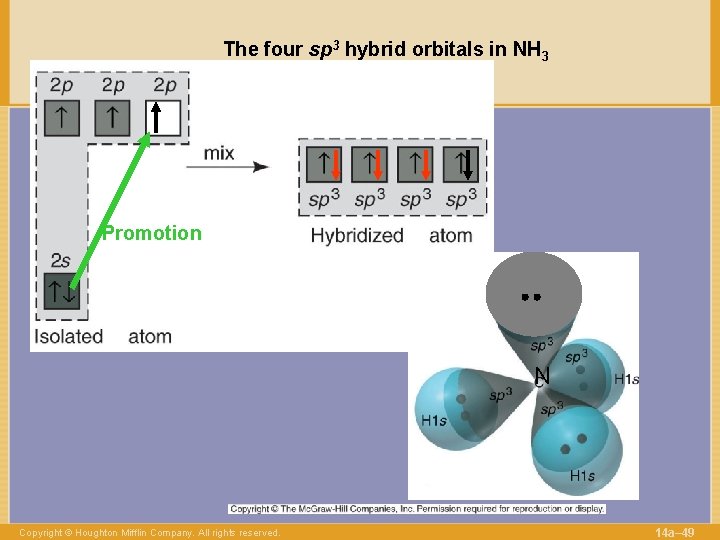
The four sp 3 hybrid orbitals in NH 3 Promotion N Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 49
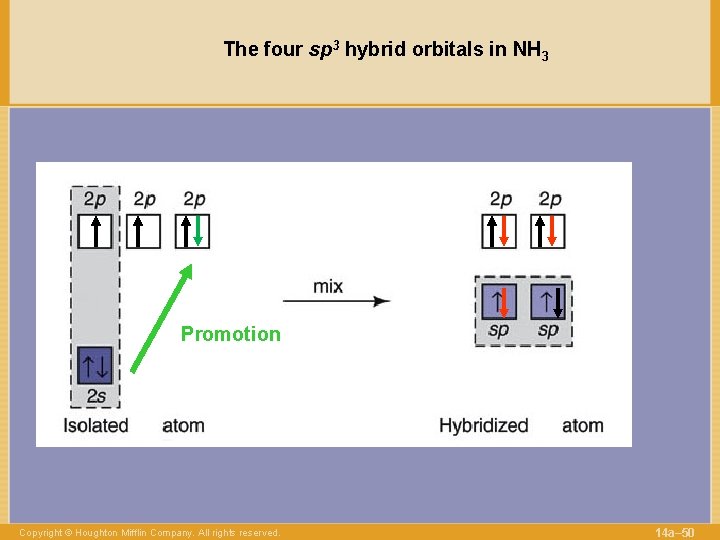
The four sp 3 hybrid orbitals in NH 3 Promotion Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 50
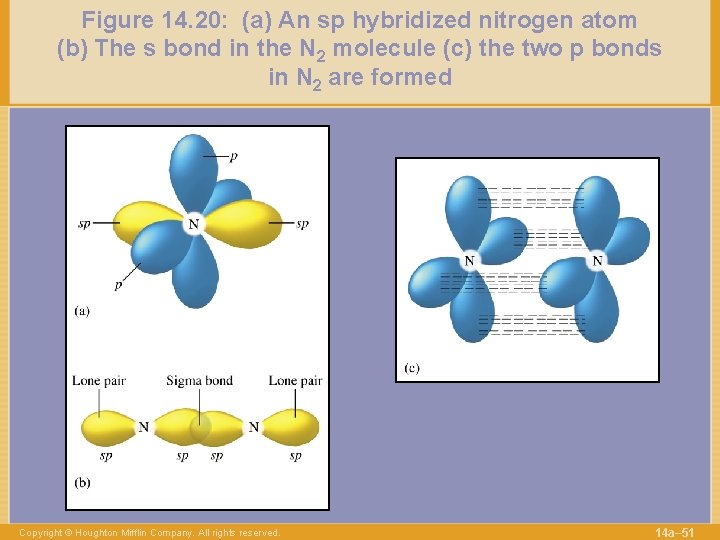
Figure 14. 20: (a) An sp hybridized nitrogen atom (b) The s bond in the N 2 molecule (c) the two p bonds in N 2 are formed Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 51
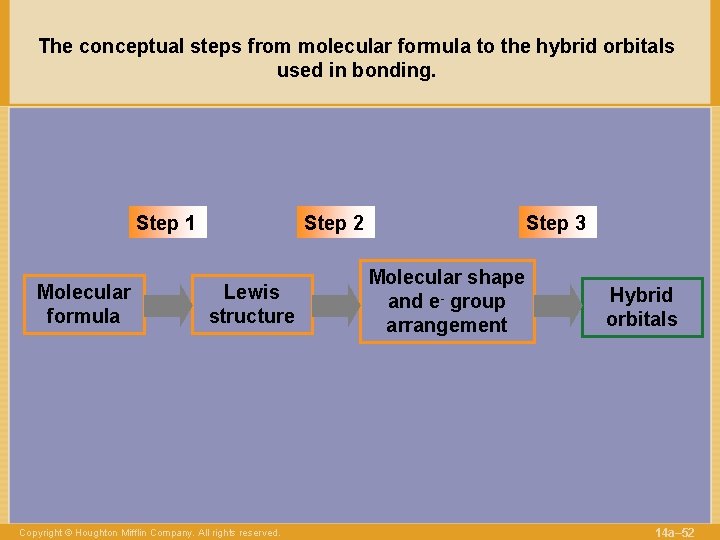
The conceptual steps from molecular formula to the hybrid orbitals used in bonding. Step 1 Molecular formula Step 2 Lewis structure Copyright © Houghton Mifflin Company. All rights reserved. Step 3 Molecular shape and e- group arrangement Hybrid orbitals 14 a– 52
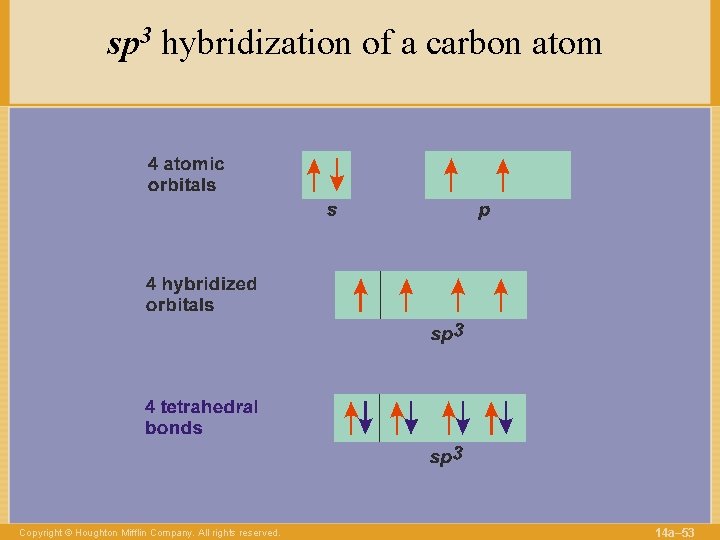
sp 3 hybridization of a carbon atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 53

sp 3 hybridization of a carbon atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 54
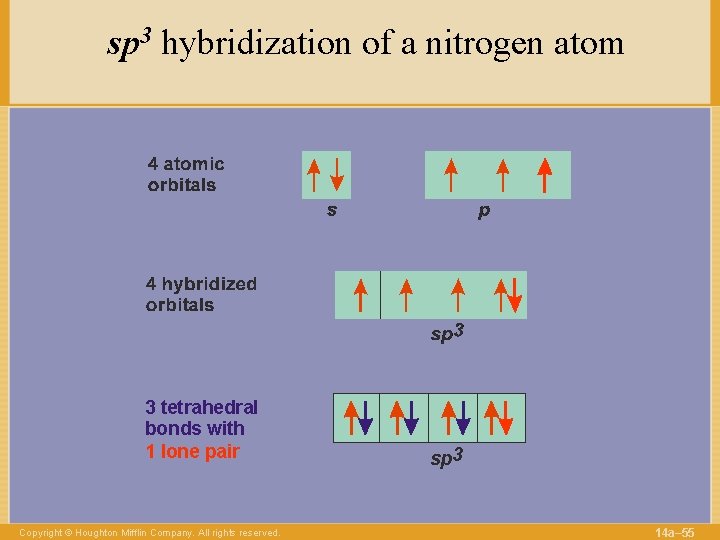
sp 3 hybridization of a nitrogen atom 3 tetrahedral bonds with 1 lone pair Copyright © Houghton Mifflin Company. All rights reserved. sp 3 14 a– 55
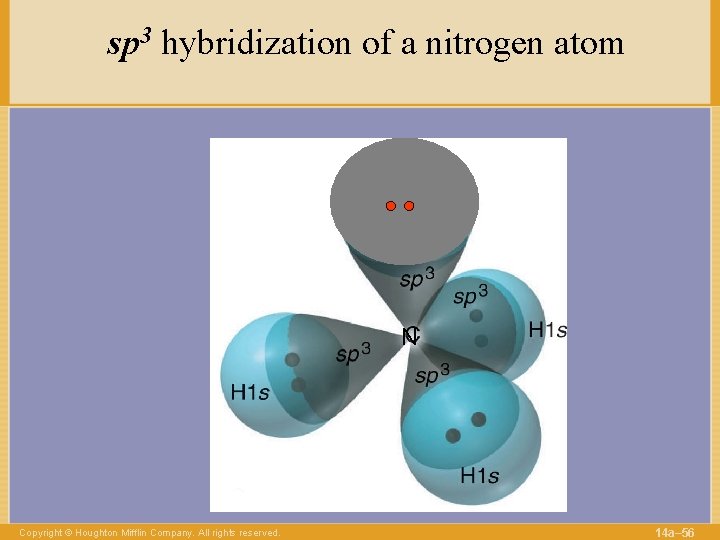
sp 3 hybridization of a nitrogen atom N Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 56
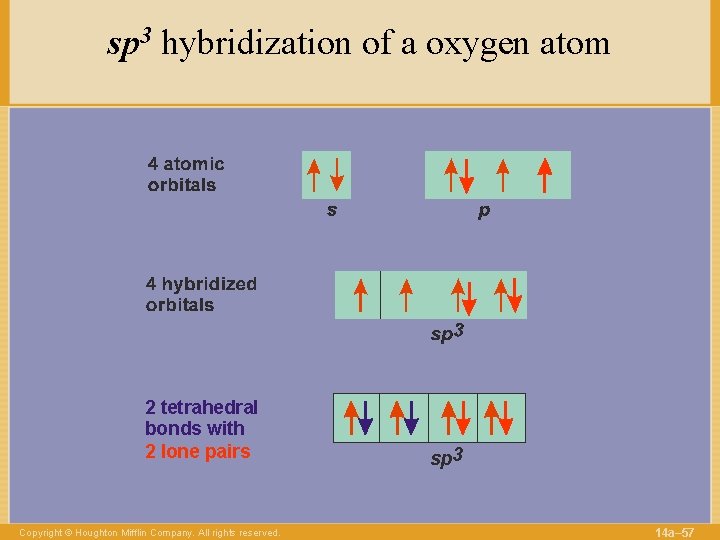
sp 3 hybridization of a oxygen atom 2 tetrahedral bonds with 2 lone pairs Copyright © Houghton Mifflin Company. All rights reserved. sp 3 14 a– 57
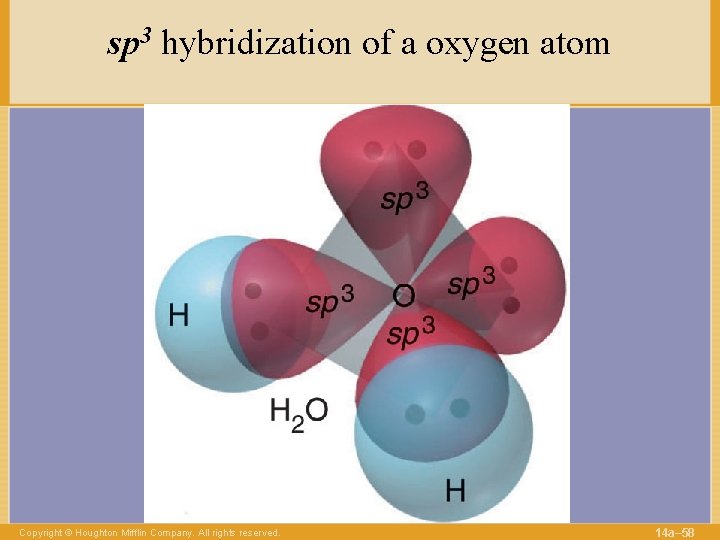
sp 3 hybridization of a oxygen atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 58
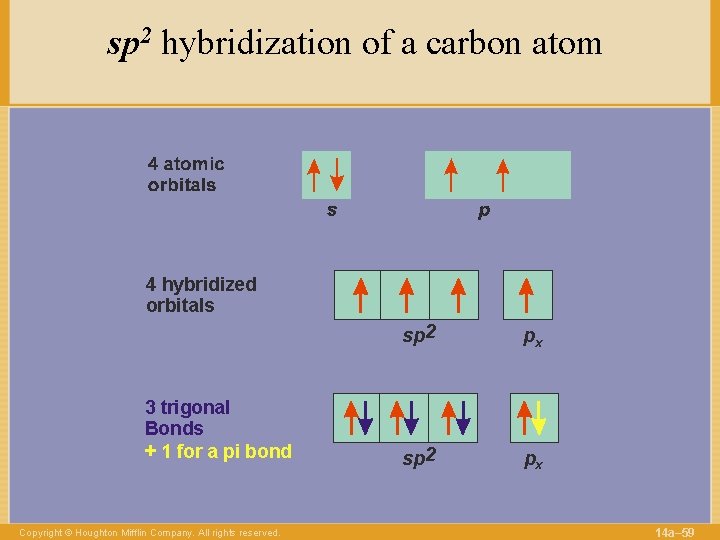
sp 2 hybridization of a carbon atom 4 hybridized orbitals 3 trigonal Bonds + 1 for a pi bond Copyright © Houghton Mifflin Company. All rights reserved. sp 2 px 14 a– 59
Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 60
Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 61
Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 62
Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 63
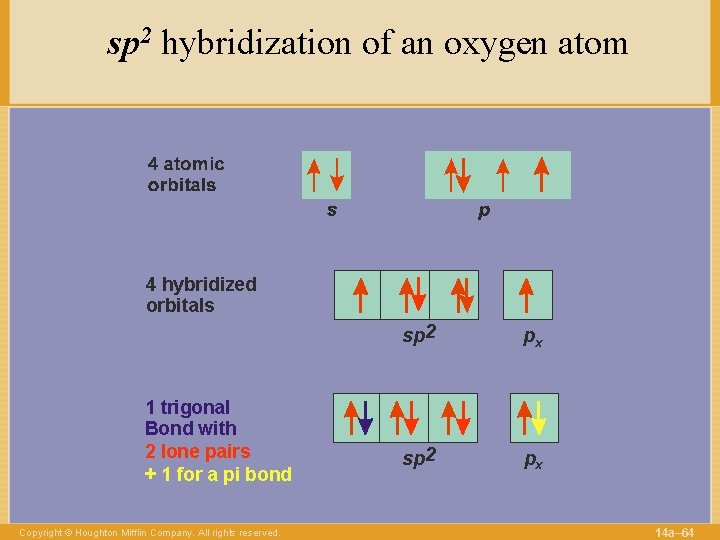
sp 2 hybridization of an oxygen atom 4 hybridized orbitals 1 trigonal Bond with 2 lone pairs + 1 for a pi bond Copyright © Houghton Mifflin Company. All rights reserved. sp 2 px 14 a– 64
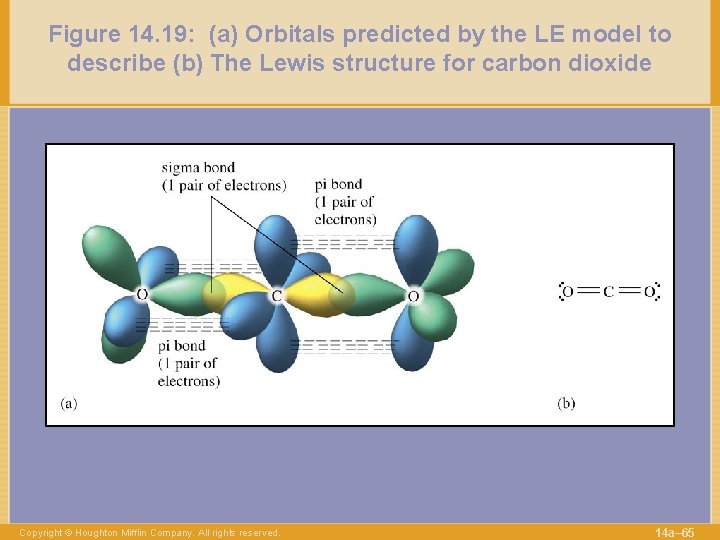
Figure 14. 19: (a) Orbitals predicted by the LE model to describe (b) The Lewis structure for carbon dioxide Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 65
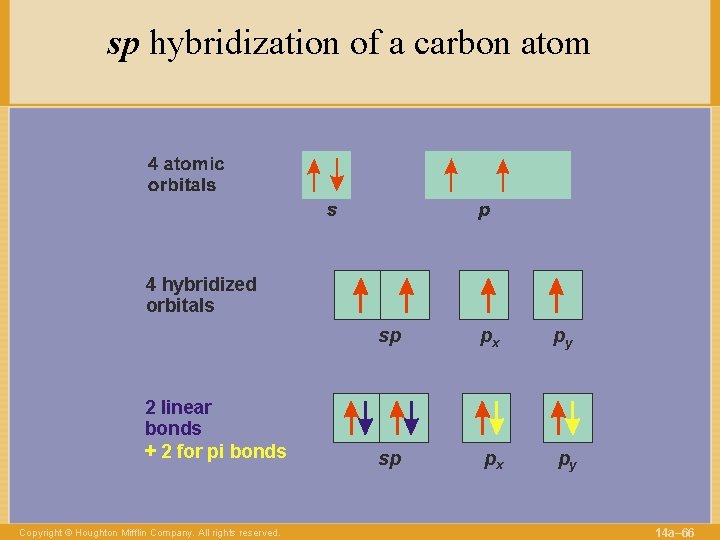
sp hybridization of a carbon atom 4 hybridized orbitals 2 linear bonds + 2 for pi bonds Copyright © Houghton Mifflin Company. All rights reserved. sp px py 14 a– 66
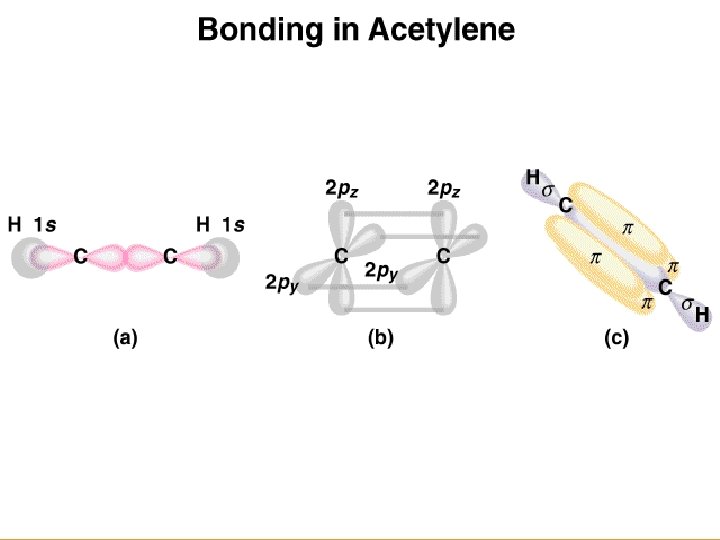
Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 67
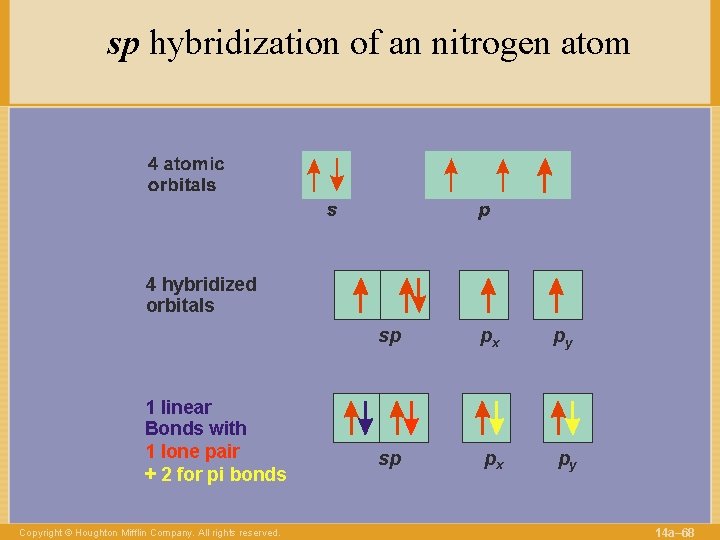
sp hybridization of an nitrogen atom 4 hybridized orbitals 1 linear Bonds with 1 lone pair + 2 for pi bonds Copyright © Houghton Mifflin Company. All rights reserved. sp px py 14 a– 68
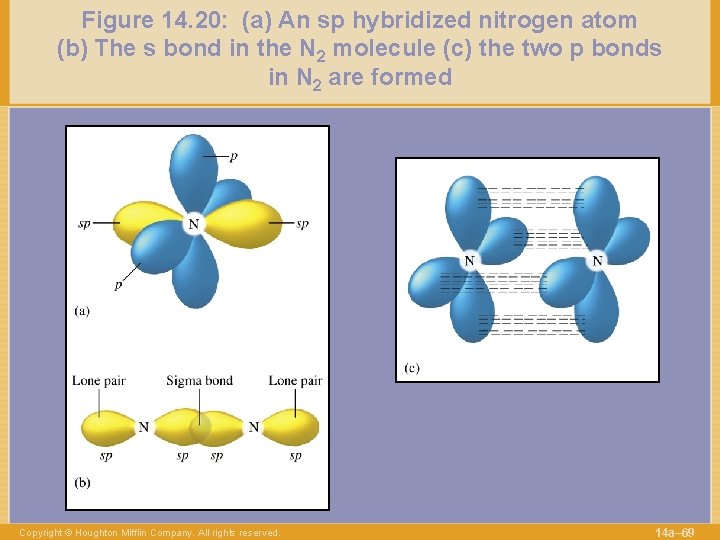
Figure 14. 20: (a) An sp hybridized nitrogen atom (b) The s bond in the N 2 molecule (c) the two p bonds in N 2 are formed Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 69
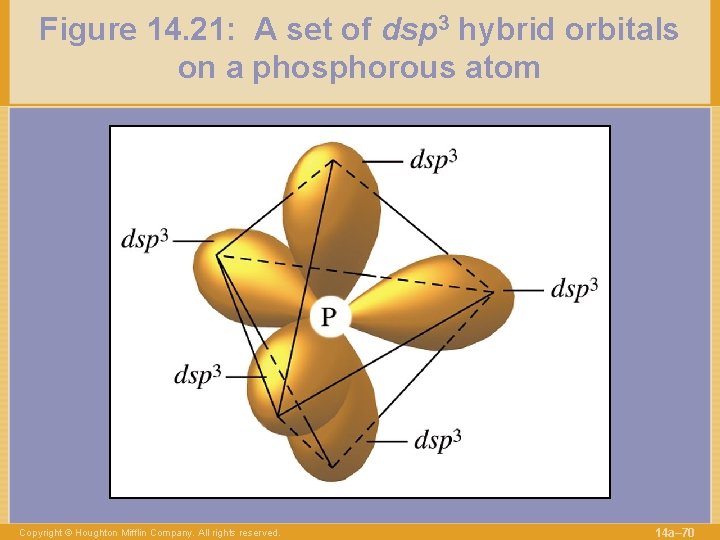
Figure 14. 21: A set of dsp 3 hybrid orbitals on a phosphorous atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 70
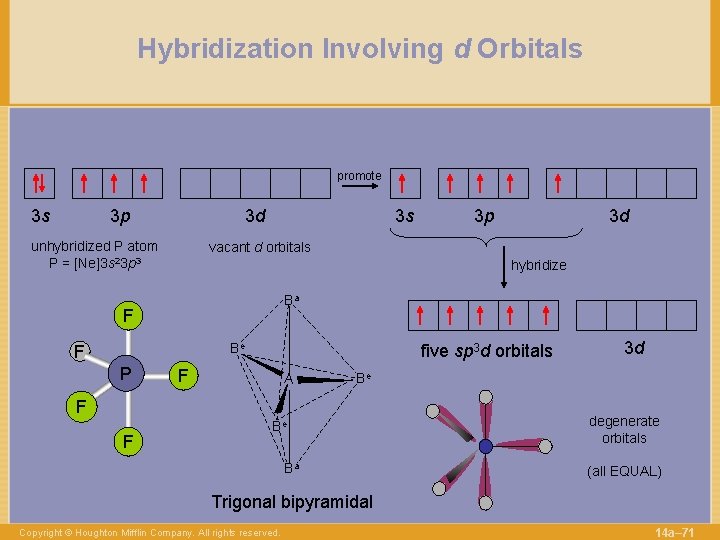
Hybridization Involving d Orbitals promote 3 s 3 p 3 d unhybridized P atom P = [Ne]3 s 23 p 3 3 s hybridize Ba Be F five sp 3 d orbitals F A 3 d Be F F 3 d vacant d orbitals F P 3 p Be Ba degenerate orbitals (all EQUAL) Trigonal bipyramidal Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 71
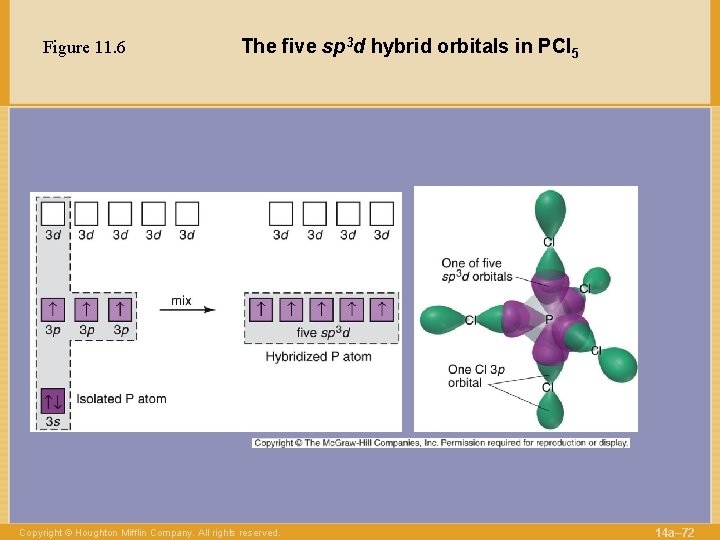
Figure 11. 6 The five sp 3 d hybrid orbitals in PCl 5 Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 72
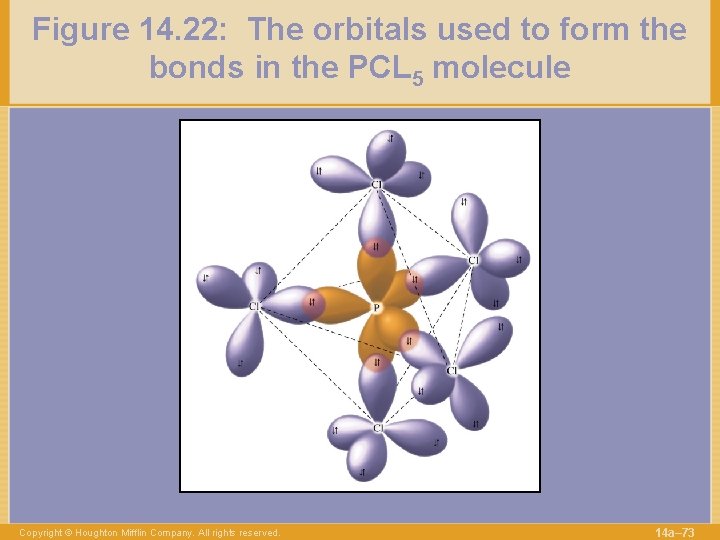
Figure 14. 22: The orbitals used to form the bonds in the PCL 5 molecule Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 73
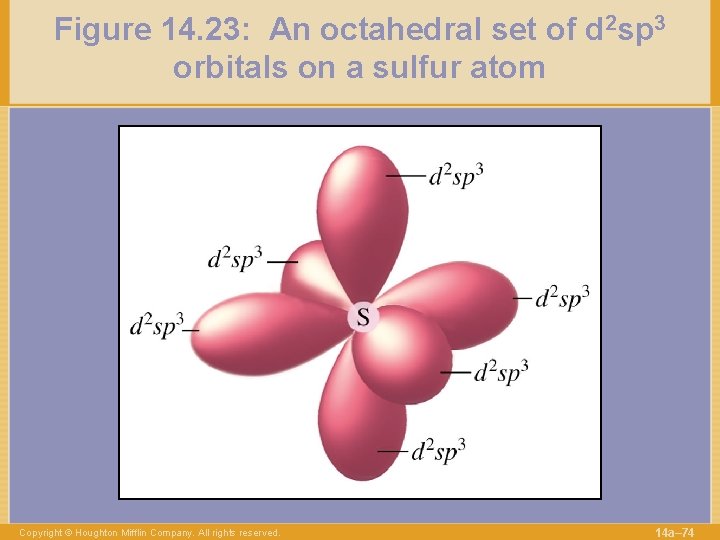
Figure 14. 23: An octahedral set of d 2 sp 3 orbitals on a sulfur atom Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 74
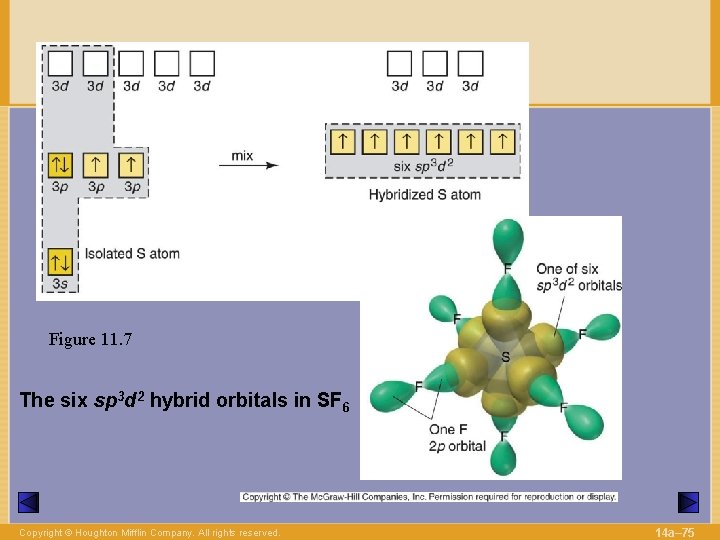
Figure 11. 7 The six sp 3 d 2 hybrid orbitals in SF 6 Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 75
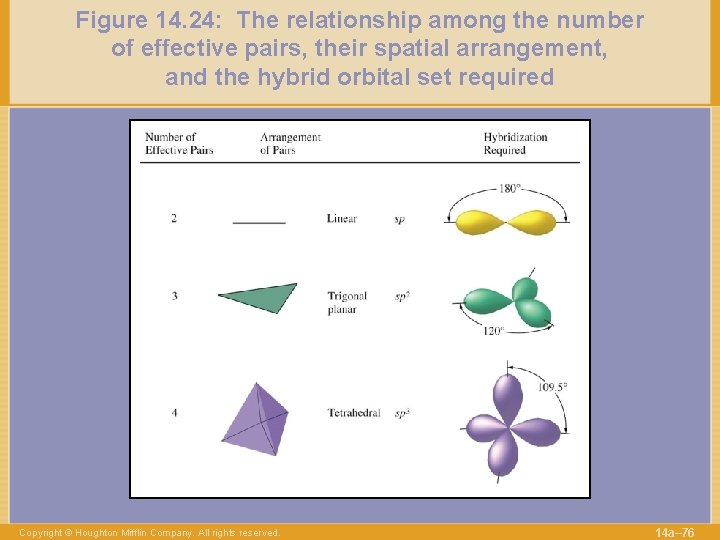
Figure 14. 24: The relationship among the number of effective pairs, their spatial arrangement, and the hybrid orbital set required Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 76
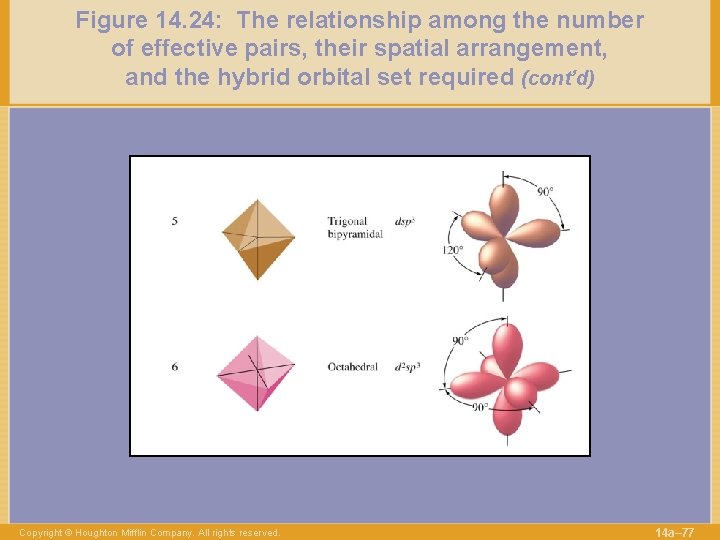
Figure 14. 24: The relationship among the number of effective pairs, their spatial arrangement, and the hybrid orbital set required (cont’d) Copyright © Houghton Mifflin Company. All rights reserved. 14 a– 77
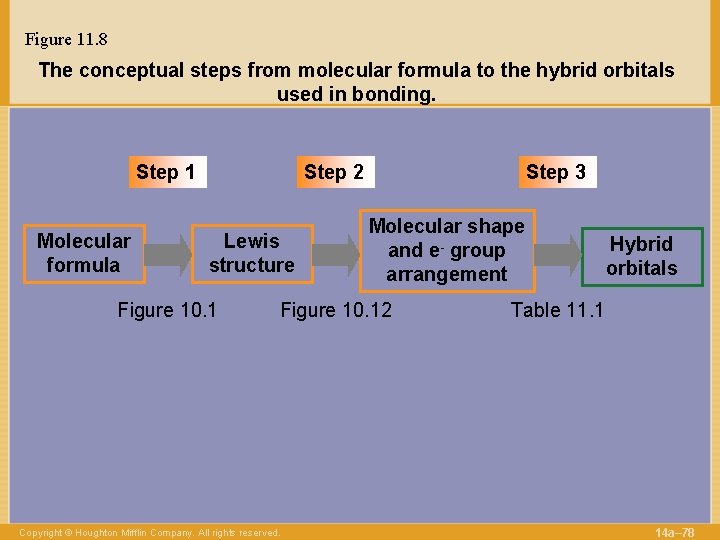
Figure 11. 8 The conceptual steps from molecular formula to the hybrid orbitals used in bonding. Step 1 Molecular formula Step 2 Lewis structure Figure 10. 1 Step 3 Molecular shape and e- group arrangement Figure 10. 12 Copyright © Houghton Mifflin Company. All rights reserved. Hybrid orbitals Table 11. 1 14 a– 78
- Slides: 78