Covalent Bonding Learning to Cooperate describe a covalent
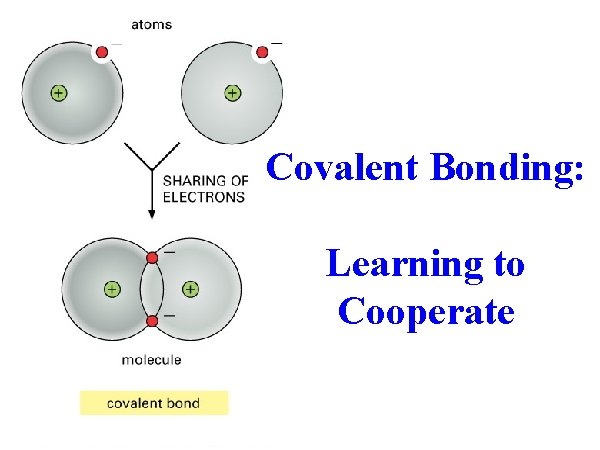
Covalent Bonding: Learning to Cooperate

• • describe a covalent bond state the differences covalent and ionic bonds explain the term molecule describe a diatomic molecule Key Words covalent compound covalent bond diatomic molecule

Covalent (Molecular) Compound: Contains two or more NON-METAL atoms. Formed by SHARING valence electrons to fill outer shell – octet rule. *A molecule is the smallest unit of a covalent compound. * non-metal + non-metal = covalent bond
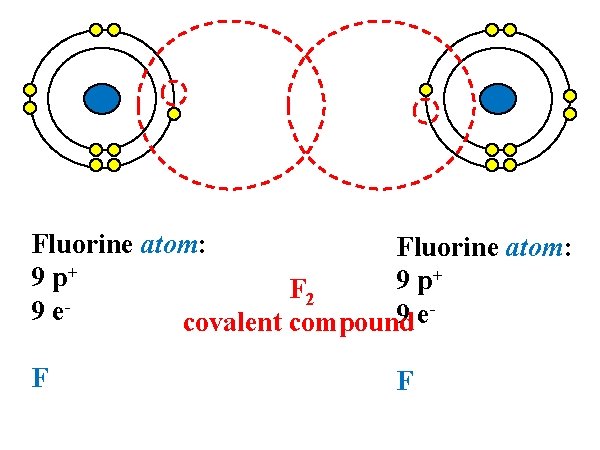
Fluorine atom: + 9 p F 2 9 e 9 e covalent compound F F
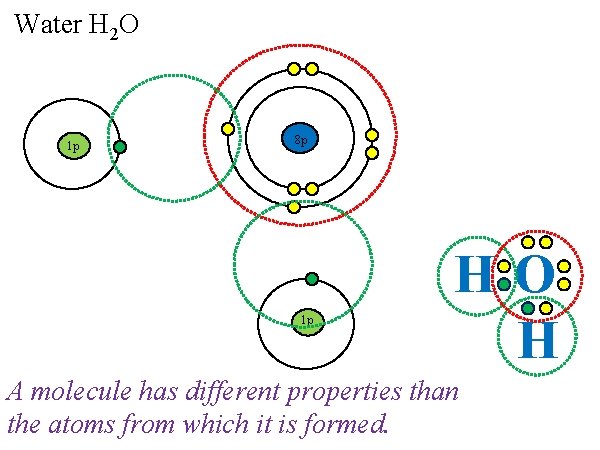
Water H 2 O 1 p 8 p 1 p HO H A molecule has different properties than the atoms from which it is formed.
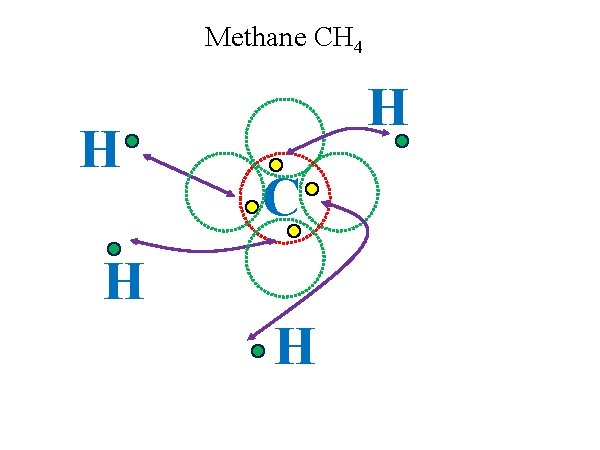
Methane CH 4 H H C H H
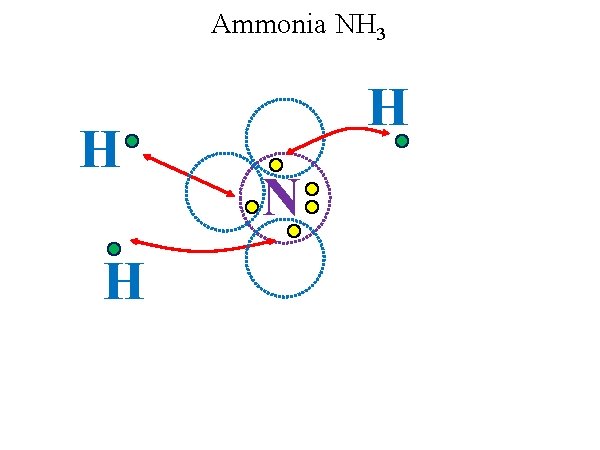
Ammonia NH 3 H H H N
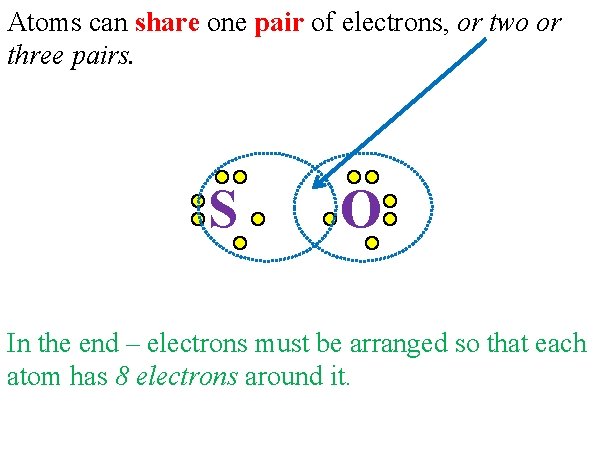
Atoms can share one pair of electrons, or two or three pairs. S O In the end – electrons must be arranged so that each atom has 8 electrons around it.
Diatomic Elements Forming a Covalent Bond with Yourself
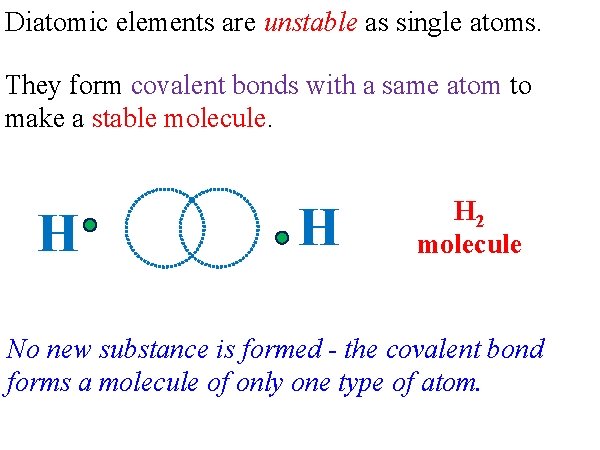
Diatomic elements are unstable as single atoms. They form covalent bonds with a same atom to make a stable molecule. H H H 2 molecule No new substance is formed - the covalent bond forms a molecule of only one type of atom.

Diatomic Elements: I H ave N o Br ight 2 2 2 O r Cl ever Friends 2 2
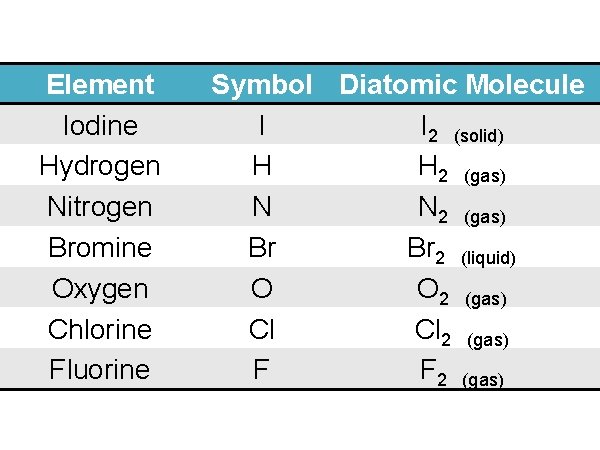
Element Iodine Hydrogen Nitrogen Bromine Oxygen Chlorine Fluorine Symbol Diatomic Molecule I I 2 (solid) H H 2 (gas) N N 2 (gas) Br Br 2 (liquid) O O 2 (gas) Cl Cl 2 (gas) F F 2 (gas)
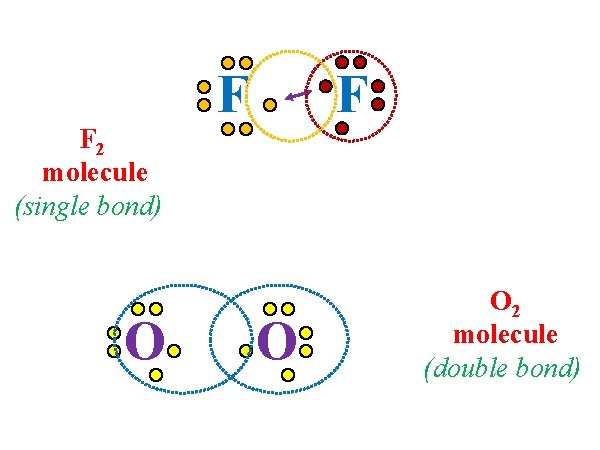
F 2 molecule (single bond) O F F O O 2 molecule (double bond)
Covalent Bonding: Naming and Writing

Same – covalent compounds are also named using the “ide” ending for last non-metal. Different – covalent naming uses prefixes before the name of each element. Prefixes show the number of atoms of that element in the formula for the molecule. 2 Diatomic Elements
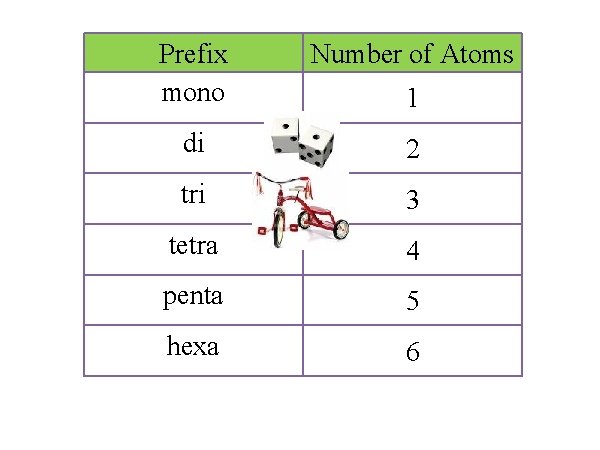
Prefix mono Number of Atoms di 2 tri 3 tetra 4 penta 5 hexa 6 1

Step 1: first non-metal is named with a prefix to show the number of atoms. We do not use “mono” for the first non-metal. Step 2: second non-metal is named with a prefix AND with the “ide” ending. N 2 O 4 di nitrogen tetra oxide dinitrogen tetroxide Prefix Number of Atoms mono 1 di 2 tri 3 tetra 4 penta 5 hexa 6
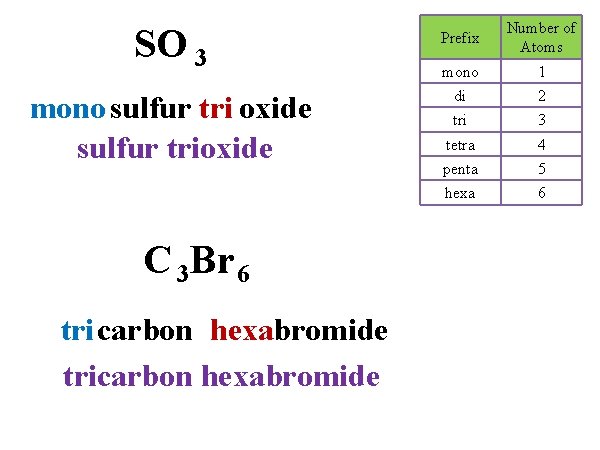
SO 3 Prefix Number of Atoms mono 1 mono sulfur tri oxide sulfur trioxide di 2 tri 3 tetra 4 penta 5 hexa 6 C 3 Br 6 tri carbon hexabromide tricarbon hexabromide
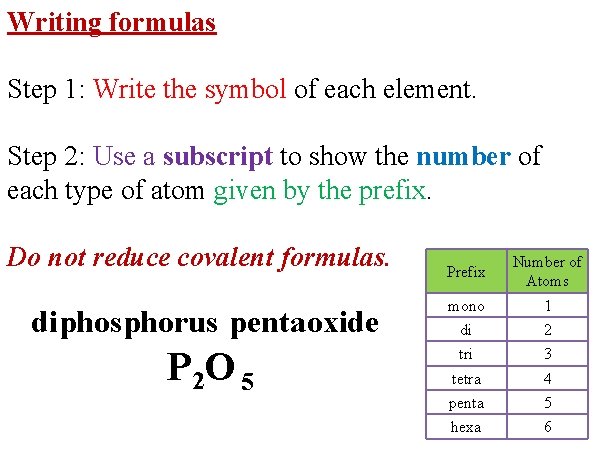
Writing formulas Step 1: Write the symbol of each element. Step 2: Use a subscript to show the number of each type of atom given by the prefix. Do not reduce covalent formulas. diphosphorus pentaoxide P 2 O 5 Prefix Number of Atoms mono 1 di 2 tri 3 tetra 4 penta 5 hexa 6
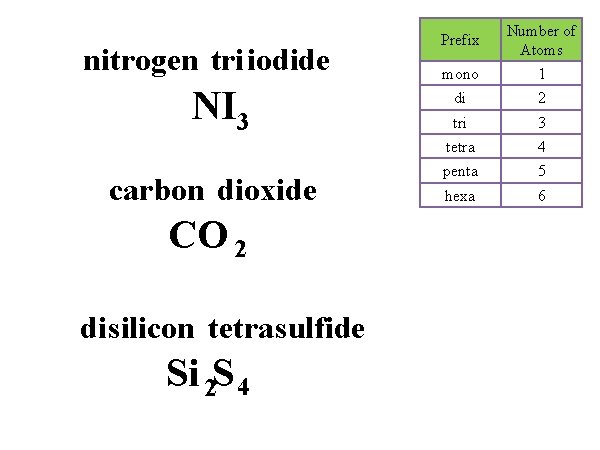
nitrogen tri iodide NI 3 carbon dioxide CO 2 di silicon tetra sulfide Si 2 S 4 Prefix Number of Atoms mono 1 di 2 tri 3 tetra 4 penta 5 hexa 6


IMPORTANT POINTS: **Always identify a compound as ionic (m + nm) or covalent (nm + nm) before doing anything. Prefixes are used in naming covalent compounds ONLY. Criss-crossing is only used in ionic compounds. DO NOT reduce covalent compounds.
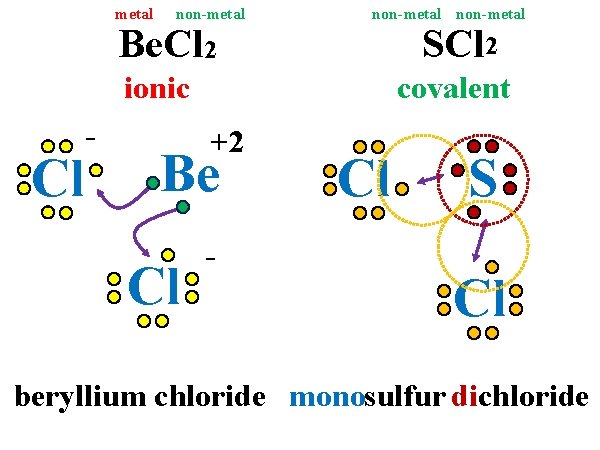
metal non-metal Be. Cl 2 non-metal SCl 2 ionic Cl - covalent +2 Be Cl - Cl S Cl beryllium chloride monosulfur dichloride
- Slides: 23