COVALENT BONDING formed from the sharing of pairs
COVALENT BONDING -formed from the sharing of pairs of electrons between atoms of nonmetallic elements - Sharing of electrons occurred to satisfy the octet requirements of the atoms
In covalent bonds, electrons are shared between electronegative elements.

Carbon-carbon covalent bonding
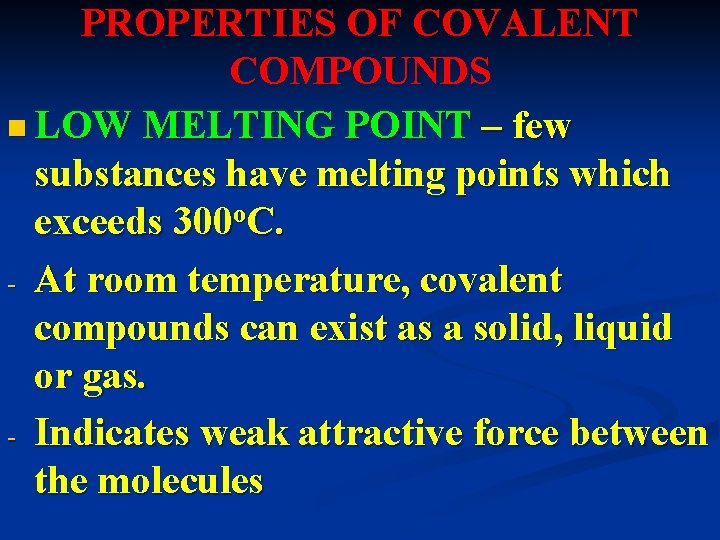
PROPERTIES OF COVALENT COMPOUNDS n LOW MELTING POINT – few substances have melting points which exceeds 300 o. C. - At room temperature, covalent compounds can exist as a solid, liquid or gas. - Indicates weak attractive force between the molecules

n SOFT SOLIDS – covalent compound solids are soft at room temperature n ELECTRICAL CONDUCTIVITY – whether solid, liquid or gas, covalent compounds are non-conductors of electricity
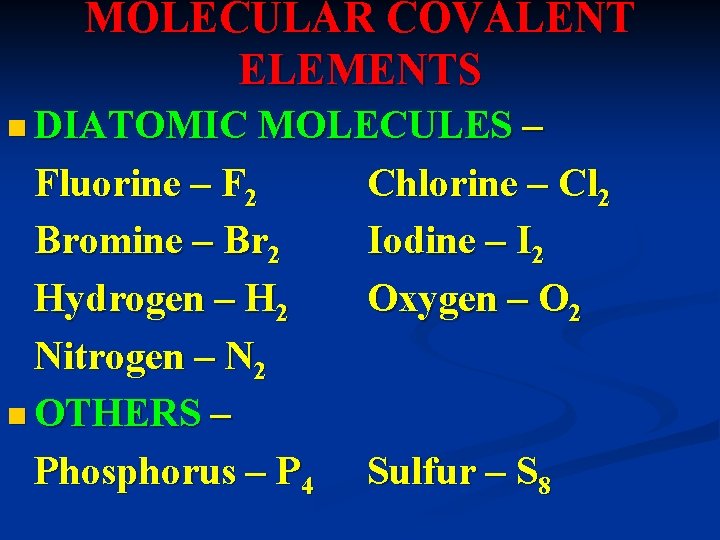
MOLECULAR COVALENT ELEMENTS n DIATOMIC MOLECULES Fluorine – F 2 Bromine – Br 2 Hydrogen – H 2 Nitrogen – N 2 n OTHERS – Phosphorus – P 4 – Chlorine – Cl 2 Iodine – I 2 Oxygen – O 2 Sulfur – S 8

n NOBLE GASES – exists as neutral atoms NOT molecules He, Ne, Ar, Kr, Xe and Rn atoms
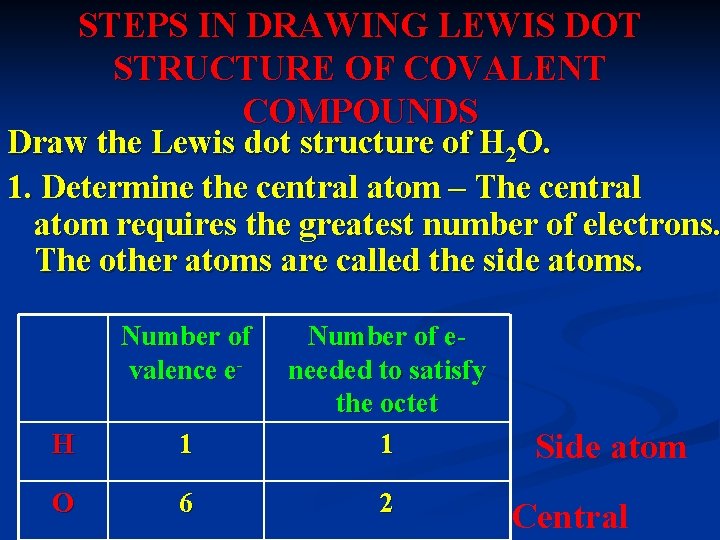
STEPS IN DRAWING LEWIS DOT STRUCTURE OF COVALENT COMPOUNDS Draw the Lewis dot structure of H 2 O. 1. Determine the central atom – The central atom requires the greatest number of electrons. The other atoms are called the side atoms. Number of valence e. H 1 Number of eneeded to satisfy the octet 1 O 6 2 Side atom Central
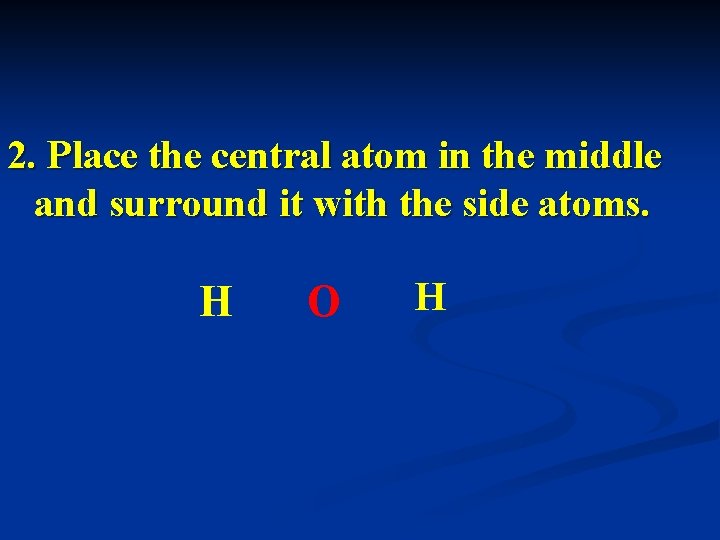
2. Place the central atom in the middle and surround it with the side atoms. H O H

3. Determine the total number of valence electrons needed to satisfy the octet. 2 H atoms x 2 e- / H atoms = 4 e 1 O atom x 8 e- / O atom = + 8 ee- needed for octet 12 e-

EXCEPTIONS TO OCTET RULE ELEMENT VALENCE e. REQUIREMENT HYDROGEN 2 BORON 6 PHOSPHORUS 10

4. Determine the total number of valence atoms available. 2 H atoms x 1 e- / H atoms = 2 e 1 O atoms x 6 e- / O atom = 6 ee- available 8 e-

5. Subtract the total number of valence electrons available from the total number of valence electrons needed to determine the number of electrons that must be shared. e- needed for octet = 12 ee- available = 8 ee- that must be shared 4 e-
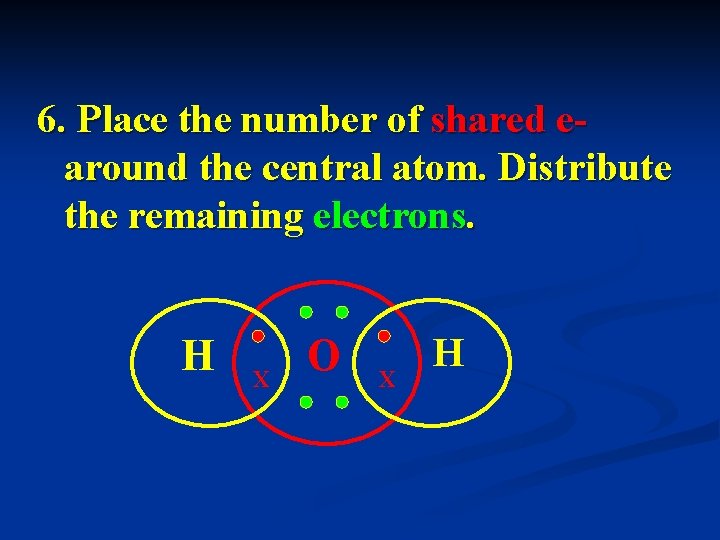
6. Place the number of shared earound the central atom. Distribute the remaining electrons. H X O X H
- Slides: 14