Covalent Bonding Covalent bonding Usually forms between two

Covalent Bonding

Covalent bonding ……. Usually forms between two nonmetals (takers) Neither want to give up their electrons but are willing to share electrons

Exception: Hydrogen • Hydrogen has one electron, but only needs one more to be “happy” Hydrogen and a nonmetal …. . neither wants to give up their electrons and neither has enough pull to take the other’s so they share a pair of electrons
Covalent Bonds form molecules Molecule = smallest part of a covalent compound
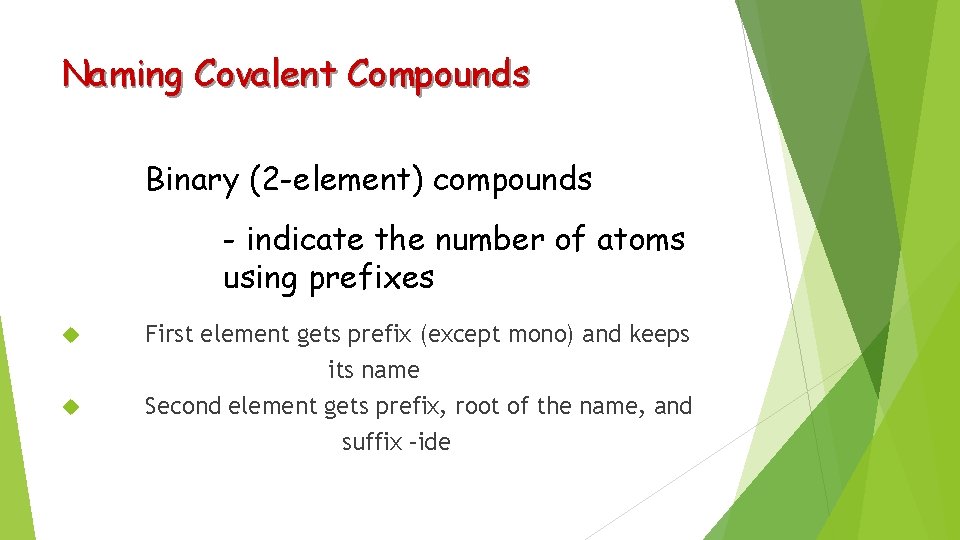
Naming Covalent Compounds Binary (2 -element) compounds - indicate the number of atoms using prefixes First element gets prefix (except mono) and keeps its name Second element gets prefix, root of the name, and suffix –ide
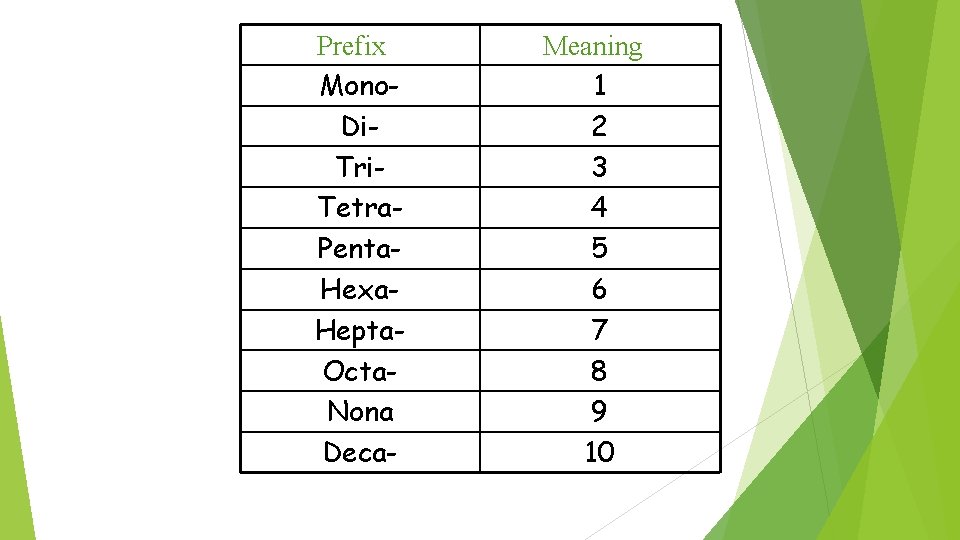
Prefix Mono. Di. Tri. Tetra. Penta. Hexa. Hepta. Octa. Nona Deca- Meaning 1 2 3 4 5 6 7 8 9 10
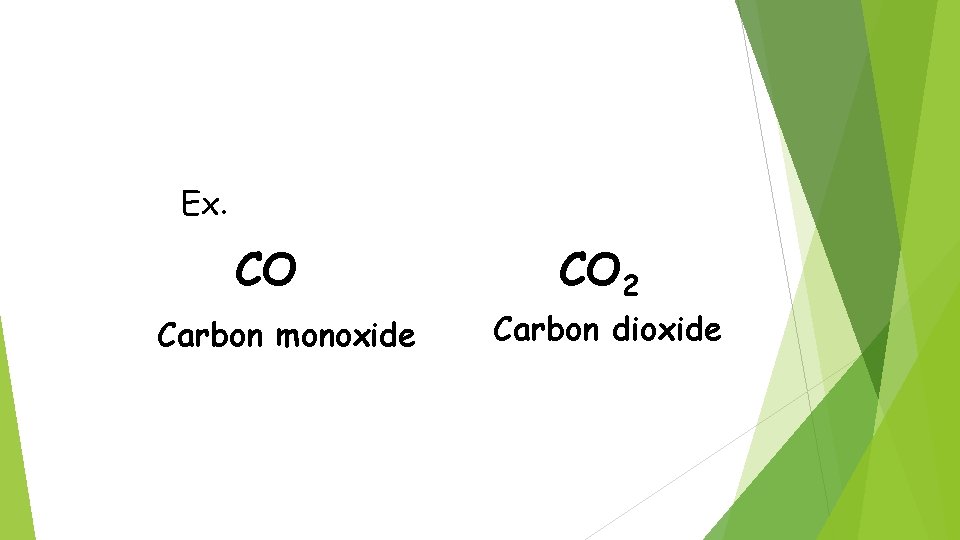
Ex. CO Carbon monoxide CO 2 Carbon dioxide

N 2 O 4 Dinitrogen tetraoxide
The number covalent bonds that will form depends on the # of valence electrons
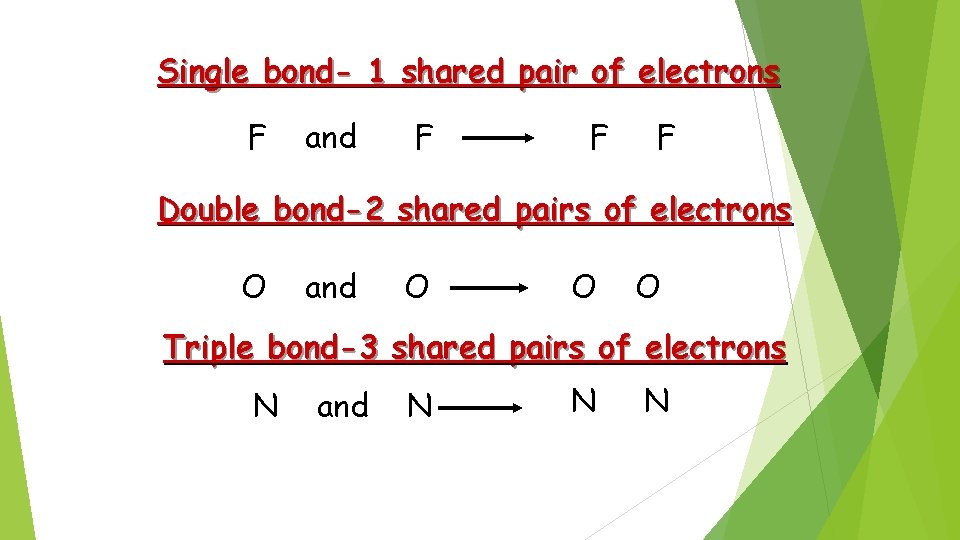
Single bond- 1 shared pair of electrons F and F F F Double bond-2 shared pairs of electrons O and O O O Triple bond-3 shared pairs of electrons N and N N N
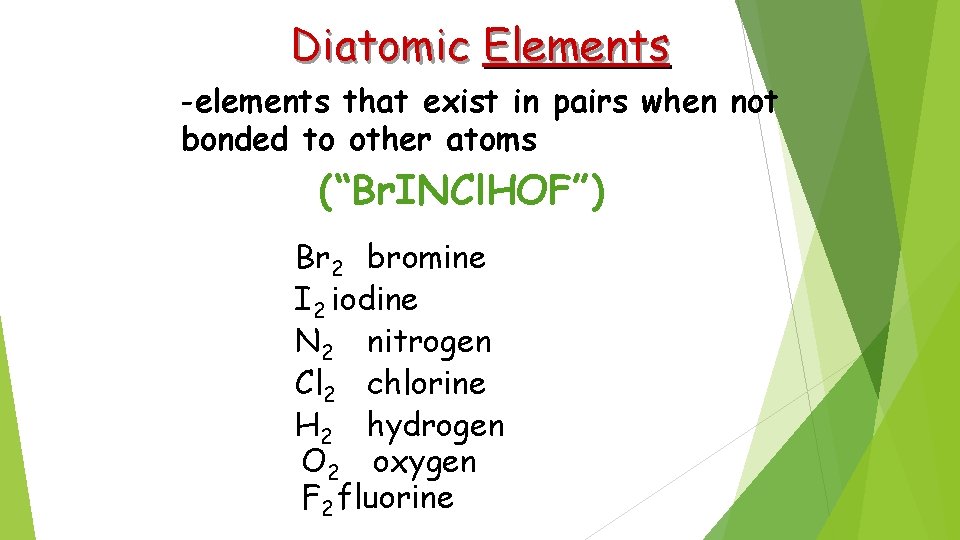
Diatomic Elements -elements that exist in pairs when not bonded to other atoms (“Br. INCl. HOF”) Br 2 bromine I 2 iodine N 2 nitrogen Cl 2 chlorine H 2 hydrogen O 2 oxygen F 2 fluorine
Bond Types Covalent bonds differ in terms of how the bonded atoms share the electrons. The character of the bonds in a given molecule depends on the kind and number of atoms joined together.
Nonpolar Covalent Bonds The bonding electrons are shared equally between the nuclei of the atoms sharing electrons Examples: The diatomic molecules electronegativity difference between the atoms is very small
Polar Covalent Bonds (Polar Bonds) A covalent bond between atoms in which the e- are shared unequally The more EN atom attracts more strongly and gains a slight (less than 1) negative charge.

The less EN atom has a slight positive charge. Example: Water Oxygen has a much higher EN value than hydrogen Oxygen will have a slight negative charge, hydrogen will have a slight positive one
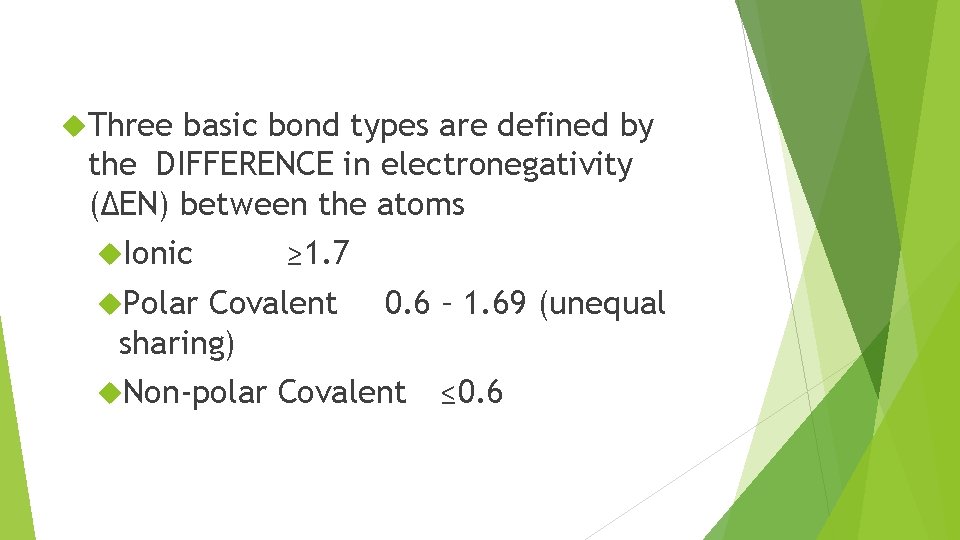
Three basic bond types are defined by the DIFFERENCE in electronegativity (ΔEN) between the atoms Ionic Polar ≥ 1. 7 Covalent sharing) Non-polar 0. 6 – 1. 69 (unequal Covalent ≤ 0. 6
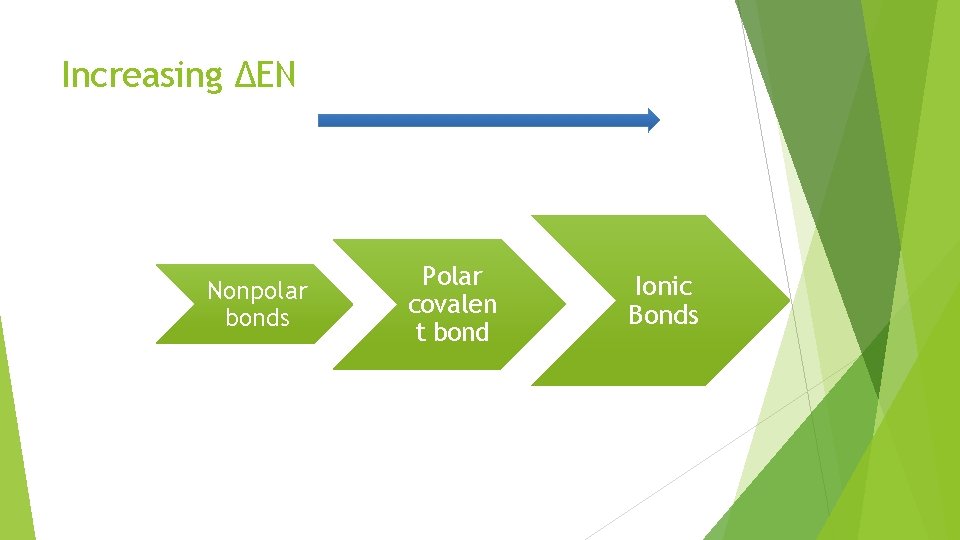
Increasing ΔEN Nonpolar bonds Polar covalen t bond Ionic Bonds
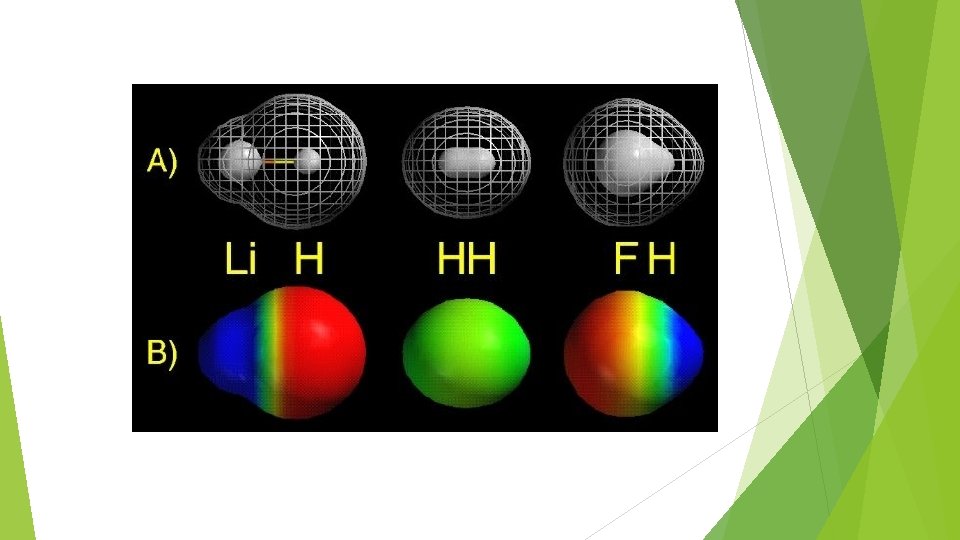
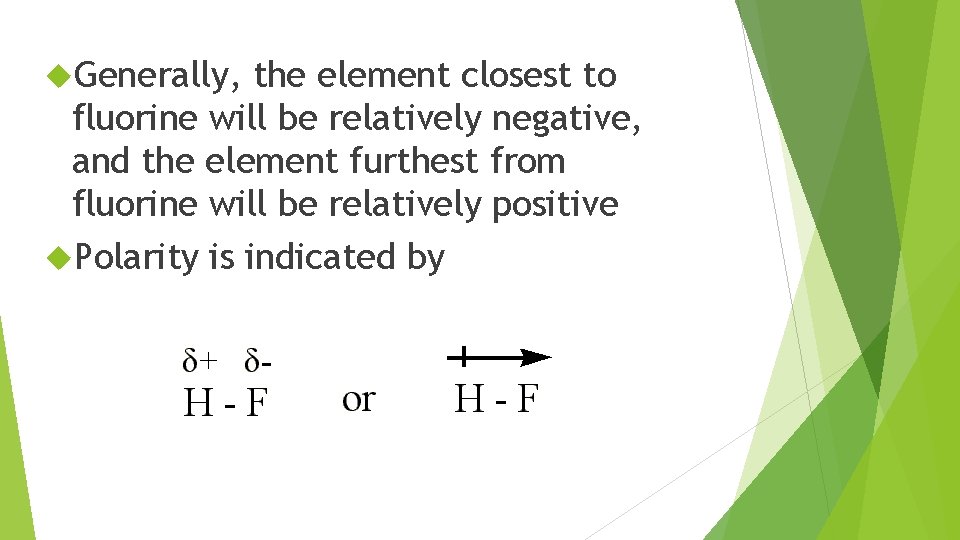
Generally, the element closest to fluorine will be relatively negative, and the element furthest from fluorine will be relatively positive Polarity is indicated by
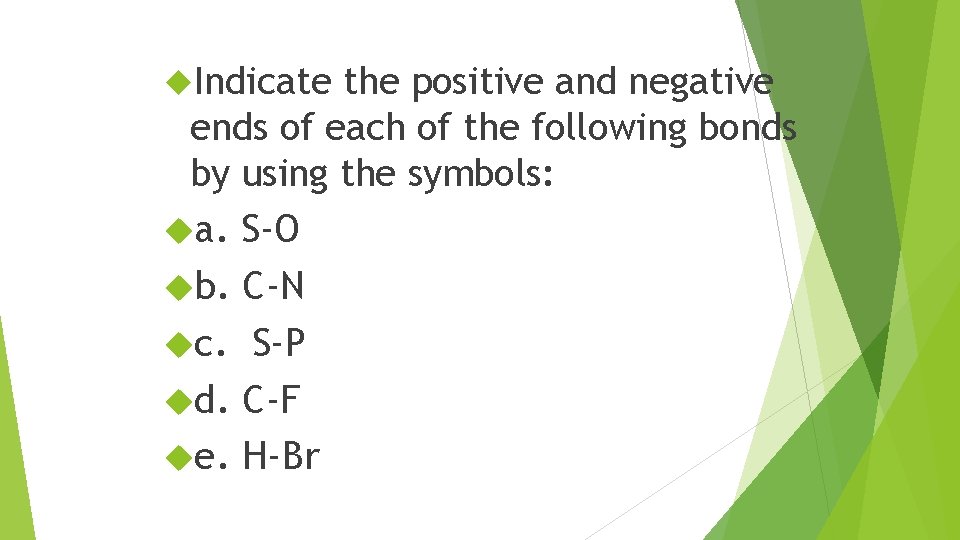
Indicate the positive and negative ends of each of the following bonds by using the symbols: a. S-O b. C-N c. S-P d. C-F e. H-Br

- Slides: 21