Covalent Bonding Covalent Bonding Recall that covalent bonding

Covalent Bonding
Covalent Bonding • Recall that covalent bonding is sharing of electrons. • It is usually a bond formed between two nonmetals • You can use electronegativities to determine all if the bond is polar covalent or nonpolar covalent • It is safe to say that nonpolar covalent bonds exist only when identical atoms are bonded to each other.

Bond Length and Bond Energy • Remember that electrons of one atom are attracted to the nucleus of the other and visa versa. • The atoms will approach each other until they reach a distance from each other that results in the possible potential energy.

Why do atoms from covalent Bonds? • Octet Rule • Since the outer shell of all noble gases (except Helium) hold eight electrons, the tendency of atoms to fill their outer energy level is the octet rule. • First look at H 2 – Notice that both H fill their outer shell if they share them equally
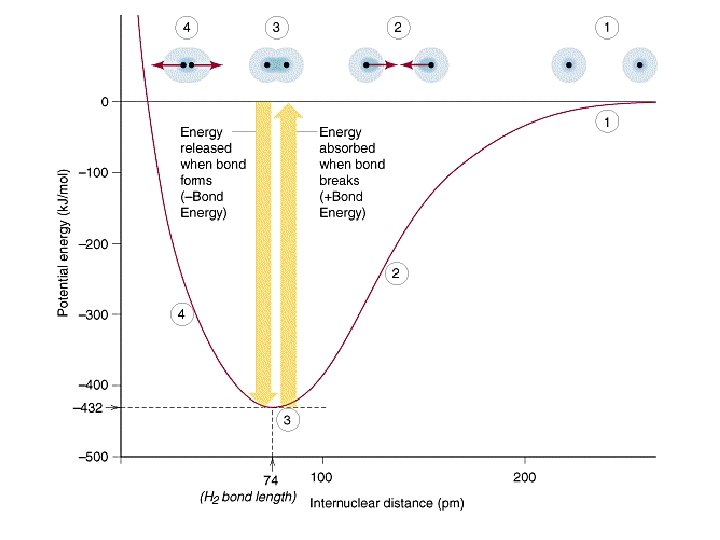

Bond Length and Bond Energy • At the minimum potential energy, the distance between the two atoms is known as the bond length • The bond energy is the amount of energy needed to break the bond, and is a measure of the strength of bond • The bond length and bond energy vary depending on the atom involved in the bond.
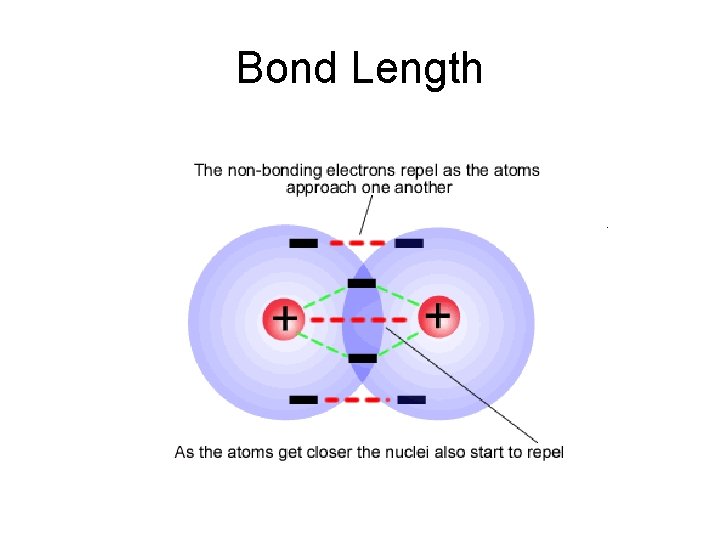
Bond Length
Bond Length • Not all bond lengths are the same • H-H bond is the shortest (0. 074 nm) • As you move down a group the bond length becomes longer • Multiple bonds are shorter than single bonds • C-C 0. 154 nm • C=C 0. 134 nm

Covalent bonds allow atoms to have the same electron conf. as noble gases • What so all the electron configurations of noble gases have in common? • Draw the bonding of two Hydrogen • Draw the bonding of two Fluorine • Draw the bonding of H-Cl


Octet Rule • All atoms want 8 electrons in the valance shell. (H – 2) • Exceptions: – Boron, which only has three valance electrons, tends to form bonds in which it is surrounded by six electrons
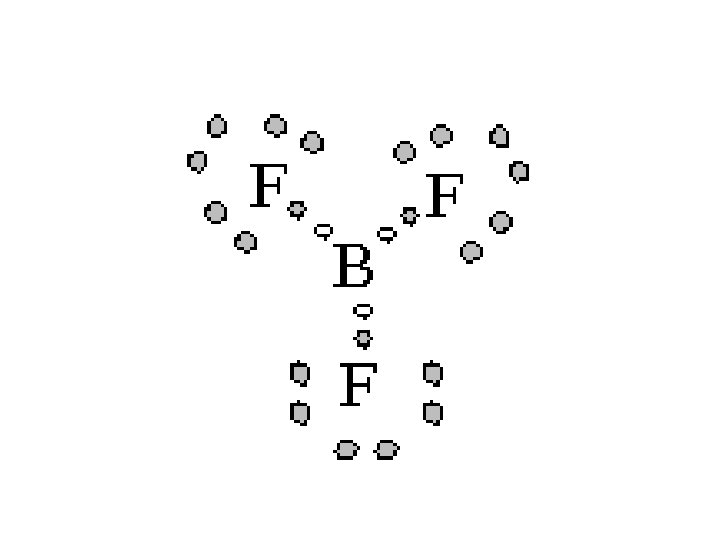

Other Exceptions • Other elements can be surrounded by more than 6 electrons by using their d orbital • Since the d orbital are used for this, only elements whose atoms have a d orbital can do this. • The first d orbital is found in the third energy level, so any atom with three or more energy levels can exceed the octet rule • Keep in mind that the octet rule is the goal, and these exceptions are possible.
Molecules • Many things around you, such as sugar, propane, and gasoline are composed of molecules • Molecules are neutral groups of atoms that are help together by covalent bonds • Compounds that consists of molecules are known as molecular compounds, or since they are held together by covalent bonds, they can be called covalent compounds
Molecules • In lab we often use Methane gas • Methane is composed of carbon and hydrogen, both of which are nonmetals, which means the bonds in methane are covalent. • Since it is held together by covalent bonds, a sample of methane gas is a molecular compound, and is made up of methane molecules.
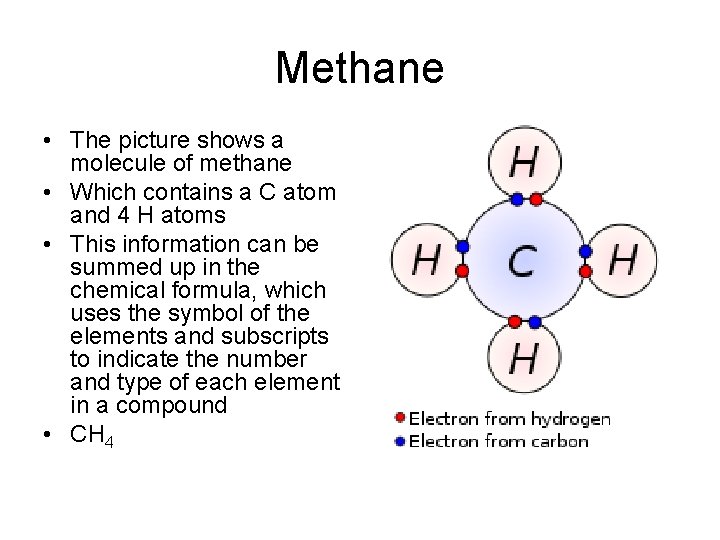
Methane • The picture shows a molecule of methane • Which contains a C atom and 4 H atoms • This information can be summed up in the chemical formula, which uses the symbol of the elements and subscripts to indicate the number and type of each element in a compound • CH 4

Review • On a sheet of paper write as much as you can on the following topics, include as many terms as possible: • Chemical bonding • Covalent bonds • Ionic bonds • Octet rule • Polar and Nonpolar • Bond Length • Molecules
- Slides: 17