Covalent Bonding Covalent Bond Atoms are held together

Covalent Bonding
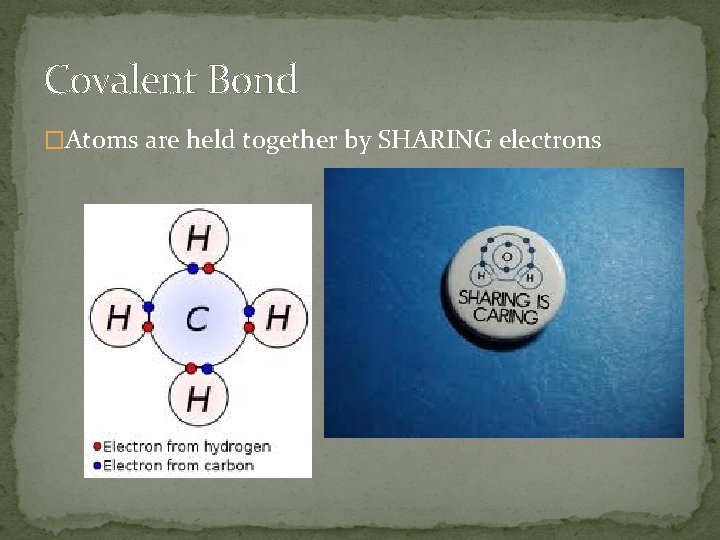
Covalent Bond �Atoms are held together by SHARING electrons
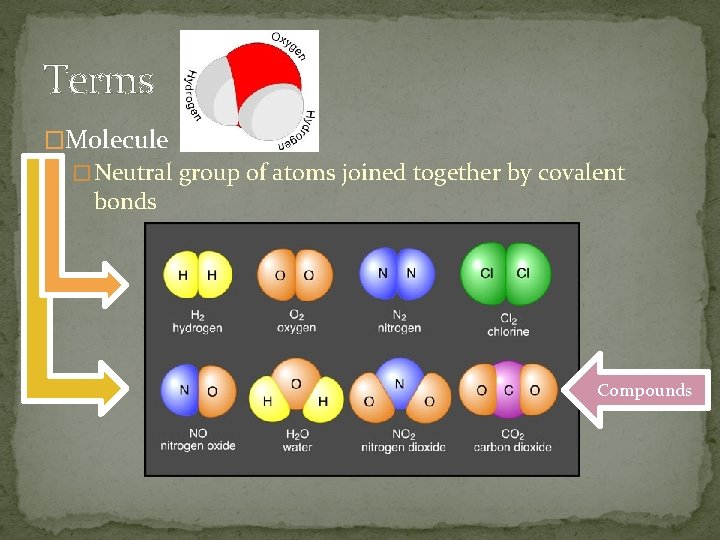
Terms �Molecule � Neutral group of atoms joined together by covalent bonds Compounds
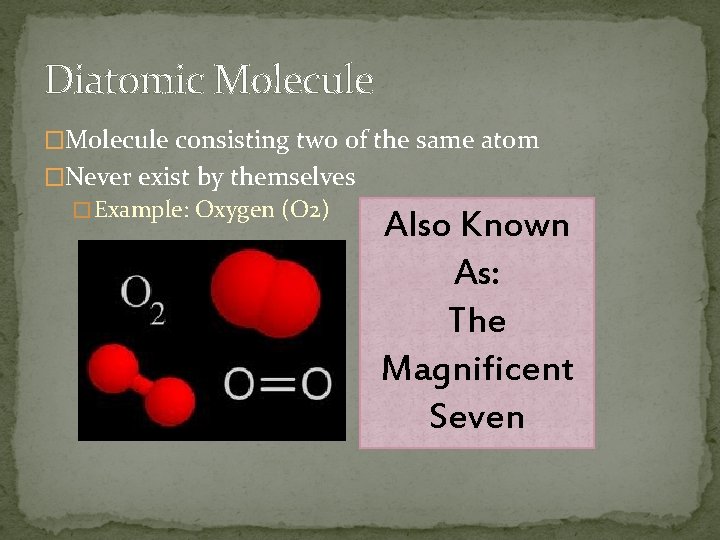
Diatomic Molecule �Molecule consisting two of the same atom �Never exist by themselves � Example: Oxygen (O 2) Also Known As: The Magnificent Seven
Diatomic Molecules N O F Cl Br I itrogen xygen lourine omine iodine

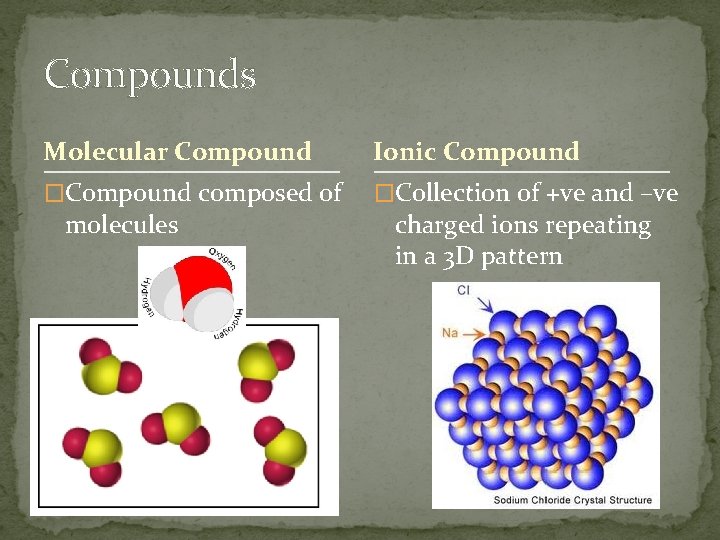
Compounds Molecular Compound Ionic Compound �Compound composed of �Collection of +ve and –ve molecules charged ions repeating in a 3 D pattern
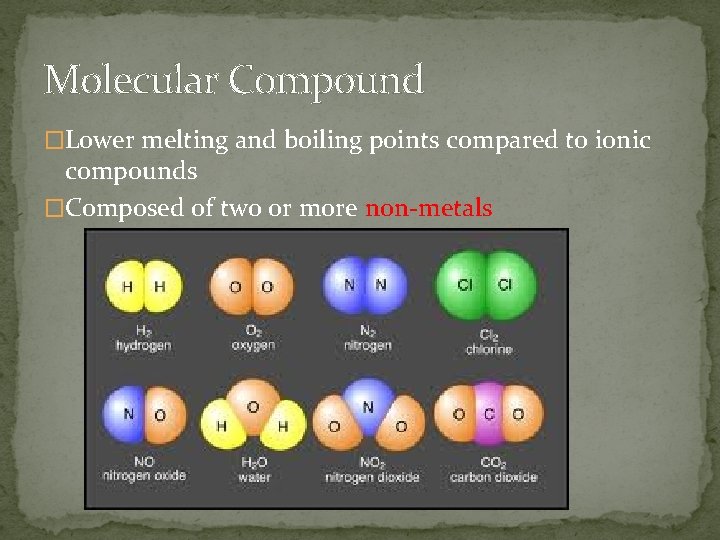
Molecular Compound �Lower melting and boiling points compared to ionic compounds �Composed of two or more non-metals

Practice Worksheet: Identifying Ionic versus Molecular Compounds

Writing Formulas for Molecular Compounds

Binary Molecular Compounds �Composed of two elements �Metal and non-metal
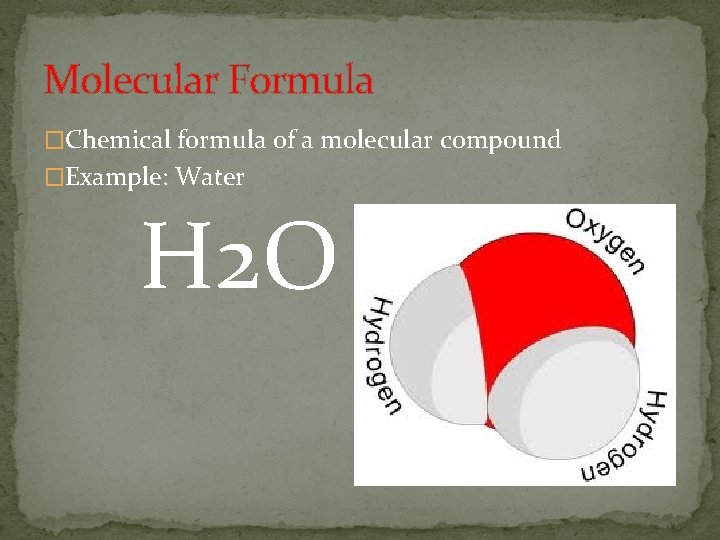
Molecular Formula �Chemical formula of a molecular compound �Example: Water H 2 O
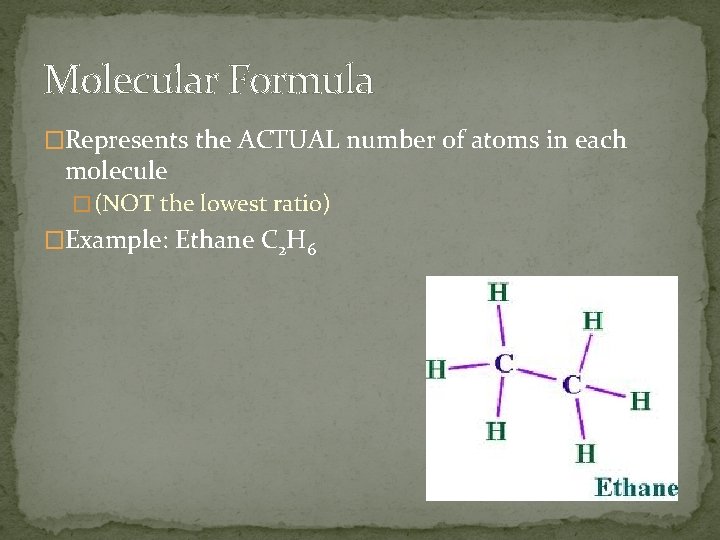
Molecular Formula �Represents the ACTUAL number of atoms in each molecule � (NOT the lowest ratio) �Example: Ethane C 2 H 6


Naming Molecular Compounds
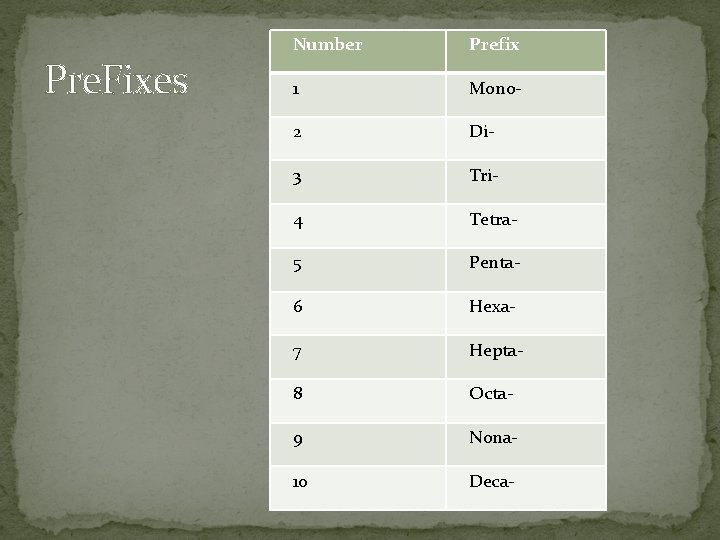
Pre. Fixes Number Prefix 1 Mono- 2 Di- 3 Tri- 4 Tetra- 5 Penta- 6 Hexa- 7 Hepta- 8 Octa- 9 Nona- 10 Deca-

Rules: �The name of a molecular compound indicates: � # of atoms � type of elements �Prefix “mono” ONLY used on the second element �For example: � CO is carbon monoxide rather than carbon oxide.

Let’s Practice �SO 2 �SF 6 �CCl 4 �NI 3

More Practice… �CF 4 �Si. O 2 �SO 3 �P 4 S 3

Practice Worksheet Naming Ionic and Covalent Bonds

The Nature of Covalent Bonding Grab a WHITEBOARD, MARKER AND TOWEL
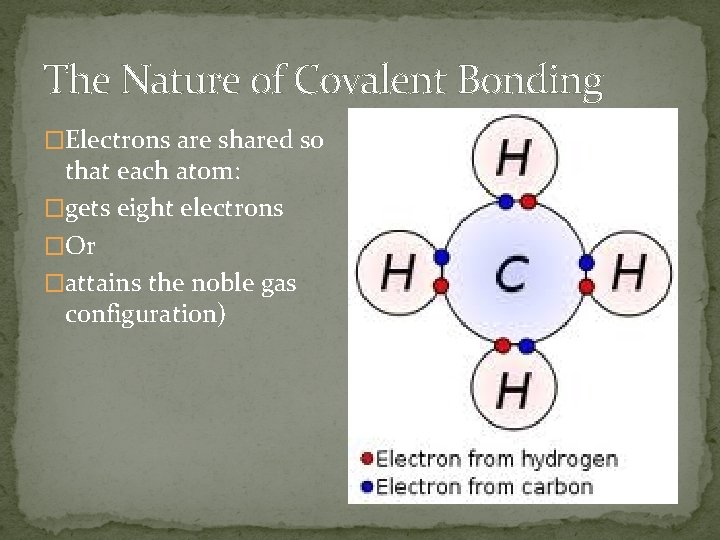
The Nature of Covalent Bonding �Electrons are shared so that each atom: �gets eight electrons �Or �attains the noble gas configuration)

Hint �The atom with the LEAST amount of valence electrons normally is in the middle… �Exception: � Hydrogen is ALWAYS on the outside �Example: HOCl

Hint �Sometimes the molecular formula tells you the arrangement of atoms �Example: CH 3 COOH

Single Covalent Bond �Two atoms held together by sharing ONE PAIR of electrons �Pair of shared e- can be represented by a dash � Structural formula �Example: �Hydrogen Molecule (H 2) �Water (H 2 O)

Unshared Pair �Not shared between atoms �Also known as: � Lone Pair � Non-Bonding �Example: A fluorine molecule (F 2)

Practice �H 2 O 2 �PCl 3

Practice �Bromine �Chlorine �Iodine
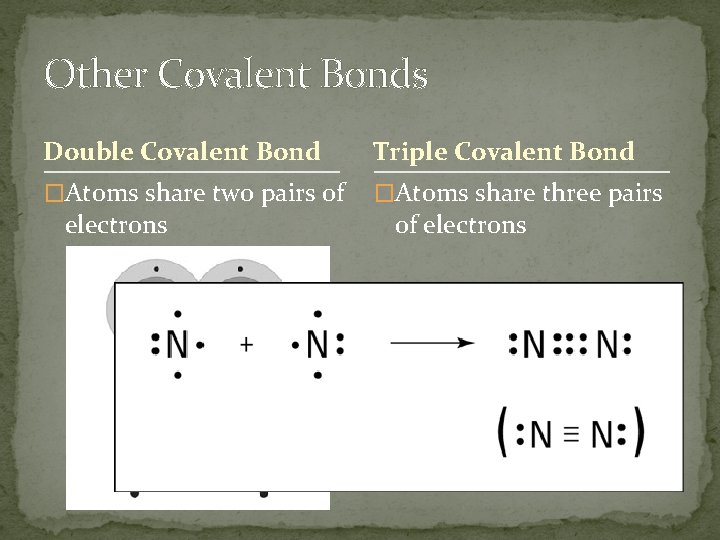
Other Covalent Bonds Double Covalent Bond Triple Covalent Bond �Atoms share two pairs of �Atoms share three pairs electrons of electrons

Double and Triple Bonds �O 2 �N 2

Double and Triple Bonds �C 2 H 6 O �NH 3

Try These… �CO �CF 4

Coordinate Covalent Bonds

Coordinate Covalent Bond �A covalent bond in which one atom contributes BOTH bonding electrons �Example: �CO 2

Coordinate Covalent Bonding �SO 3 �Cl. O 3

Try this one… �NH 4

Drawing Polyatomic Ions �REVIEW: � A tightly bound group of covalently bonded atoms that have a positive or negative charge and behave as a unit �Example: [SO 3]2 -

Polyatomic ions �Contain both covalent and coordinate covalent bonds �Electronegativity: � The ability of an atom to attract electrons when the atom is in a compound �More electronegative elements will GAIN electrons �Less electronegative elements will LOSE electrons �[OH]-
![Practice �[BF 4]- �[SO 4]2 - �[CO 3]2 - Practice �[BF 4]- �[SO 4]2 - �[CO 3]2 -](http://slidetodoc.com/presentation_image_h2/6227fe9f2ef2dc4cb707be28cbc2df59/image-39.jpg)
Practice �[BF 4]- �[SO 4]2 - �[CO 3]2 -
![Practice Problems �[BF 4]- Practice Problems �[BF 4]-](http://slidetodoc.com/presentation_image_h2/6227fe9f2ef2dc4cb707be28cbc2df59/image-40.jpg)
Practice Problems �[BF 4]-
![Practice Problems �[SO 4]2 - Practice Problems �[SO 4]2 -](http://slidetodoc.com/presentation_image_h2/6227fe9f2ef2dc4cb707be28cbc2df59/image-41.jpg)
Practice Problems �[SO 4]2 -
![Practice Problems �[CO 3]2 - Practice Problems �[CO 3]2 -](http://slidetodoc.com/presentation_image_h2/6227fe9f2ef2dc4cb707be28cbc2df59/image-42.jpg)
Practice Problems �[CO 3]2 -

Bonding Videos… �Ionic vs. Covalent Bonding
- Slides: 43