Covalent Bonding Chapter 8 Covalent bonds form when

Covalent Bonding Chapter 8 Covalent bonds form when atoms share electrons
The Covalent Bond • Atoms gain stability when they share electrons to form covalent bonds • Molecule – formed when atoms bond covalently • Covalent compounds are formed by nonmetals – Covalent: Nonmetal-Nonmetal – CNN

Properties of Covalent Compounds • • Low melting point Low boiling point Usually gas at room temperature Soft solids (candles)

Diatomic Molecules • There are SEVEN diatomic molecules • These seven elements exist at room temperature bonded together in pairs • • H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2

Naming Binary Covalent Compounds • Prefixes are used to indicate the number of atoms of each element. • -ide ending used for second element H 2 O dihydrogen monoxide • The prefix “mono” is NEVER used on the first element named. BF 3 boron trifluoride
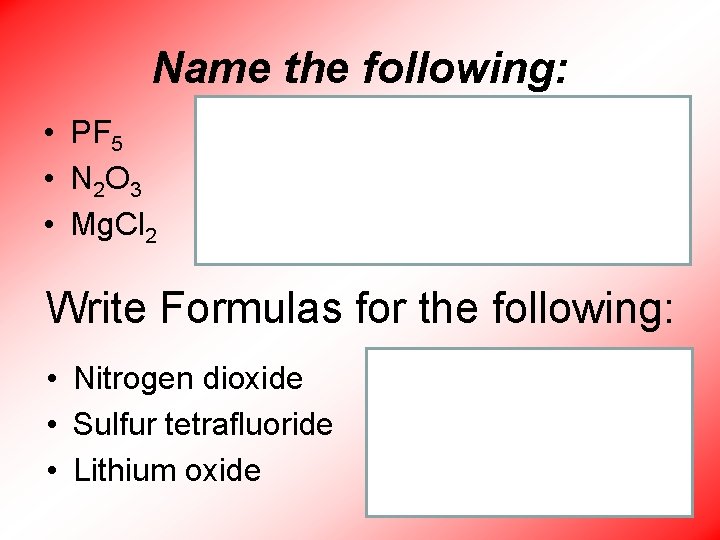
Name the following: • PF 5 • N 2 O 3 • Mg. Cl 2 • Phosphorus pentafluoride • Dinitrogen trioxide • Magnesium chloride Write Formulas for the following: • Nitrogen dioxide • Sulfur tetrafluoride • Lithium oxide • NO 2 • SF 4 • Li 2 O
Acid Nomenclature • Acids –Compounds that form H+ in water. –Formulas usually begin with ‘H’. –In order to be an acid instead of a gas, binary acids must be aqueous (dissolved in water) –Ternary acids are ALL aqueous • Examples: –HCl (aq) – hydrochloric acid –HNO 3 – nitric acid –H 2 SO 4 – sulfuric acid

• There are 2 types of acids: • Binary Acids • contains 2 elements, one being hydrogen • use hydro – prefix • change ending to – ic • the second word is always acid • Oxyacids • an acid that contains both a hydrogen atom and an oxyanion • identify the oxyanion present • the first word is the root of the oxyanion plus the suffix -ic if the anion ends in -ate or -ous if the oxyanion ends in –ite • the second word is always acid
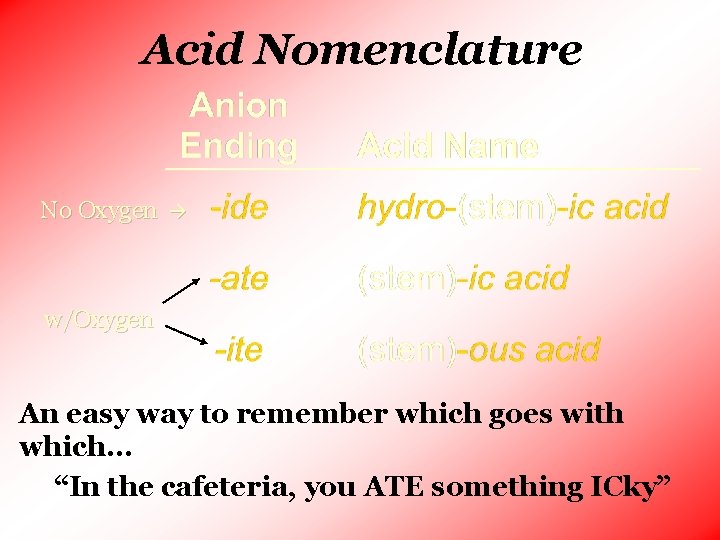
Acid Nomenclature No Oxygen w/Oxygen An easy way to remember which goes with which… “In the cafeteria, you ATE something ICky”
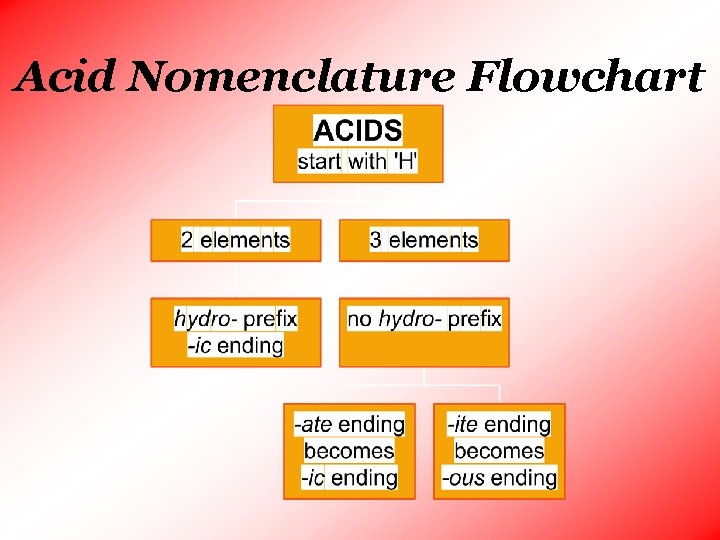
Acid Nomenclature Flowchart
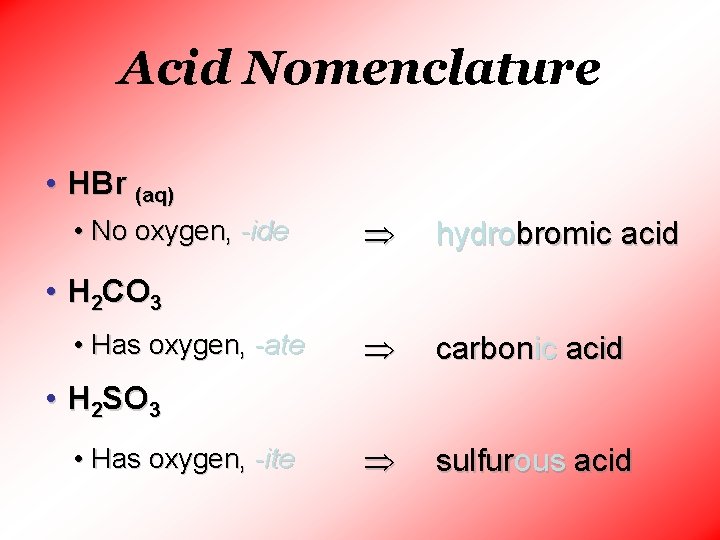
Acid Nomenclature • HBr (aq) • No oxygen, -ide hydrobromic acid carbonic acid sulfurous acid • H 2 CO 3 • Has oxygen, -ate • H 2 SO 3 • Has oxygen, -ite
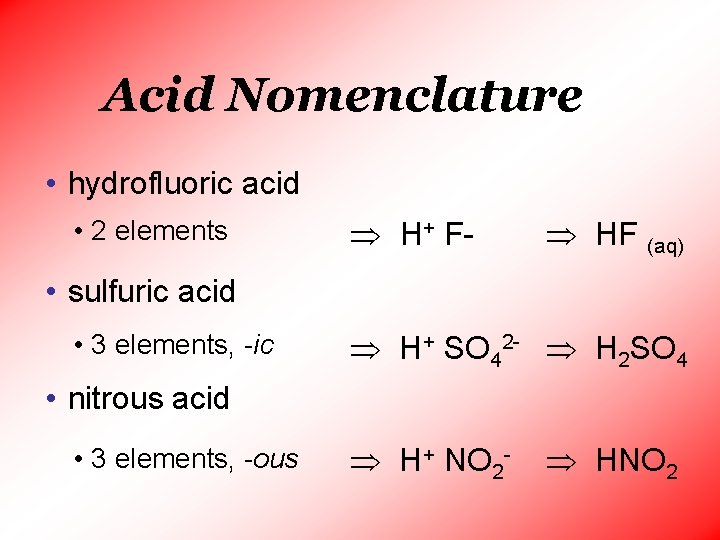
Acid Nomenclature • hydrofluoric acid • 2 elements H+ F- HF (aq) • sulfuric acid • 3 elements, -ic H+ SO 42 - H 2 SO 4 • nitrous acid • 3 elements, -ous H+ NO 2 - HNO 2
- Slides: 12