Covalent Bonding Chapter 16 Sharing electrons Two atoms

Covalent Bonding Chapter 16

Sharing electrons Two atoms share one pair of electrons Structural formulas: shows arrangement H-H: single dash shows sharing of two electrons Single covalent bonds
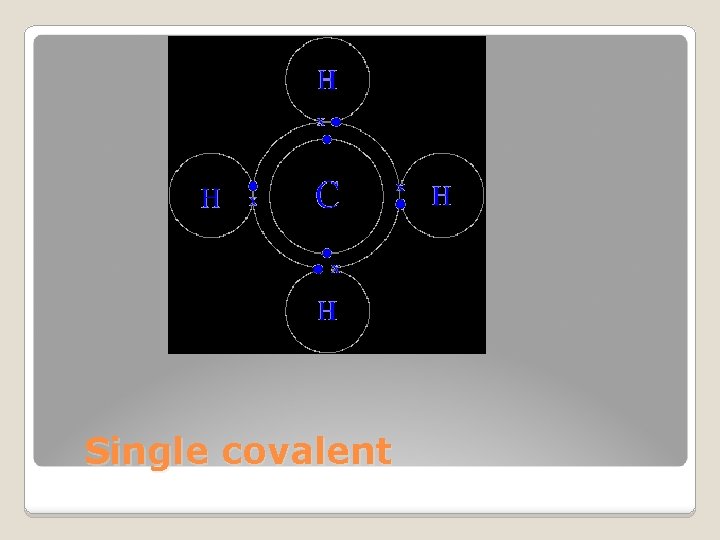
Single covalent

Electrons are shared to form an octet Gain configuration of a noble gas Two or more non metals Electron configurations

Hydrogen, nitrogen, oxygen, and the halogens (diatomic seven) Example: H 2, F 2 Diatomic molecules
Double bond: two pairs of shared electrons Triple bond: three pairs of shared electrons Double and Triple bonds

Add up valence electrons for each atom central atom is the first atom in the formula Draw a dash between covalently bonded atoms Each dash represents 2 electrons, draw the remaining electrons If not enough electrons to give an octet, change single bonds to double or triple bonds Writing dot structures for covalent compounds pg. 441
Draw the dot structure for KCl Draw the dot structure for CH 4 Draw the dot structure for CO 2 Total number of lines + individual dots = group number (# of valence) Example of dot diagram for covalent bonds

Single bond: longest length, weakest Triple bond: shortest length, strongest (requires the most energy to break) Bond Energies

Nonpolar coavlent: bonding electrons are shared equally (2 penguins or 2 polar bears) Example: H-H, O-O, N-N Polar bond: two atoms of different elements, electrons are shared unequally (polar bear and a penguin) Example: O-H Polar Bonds
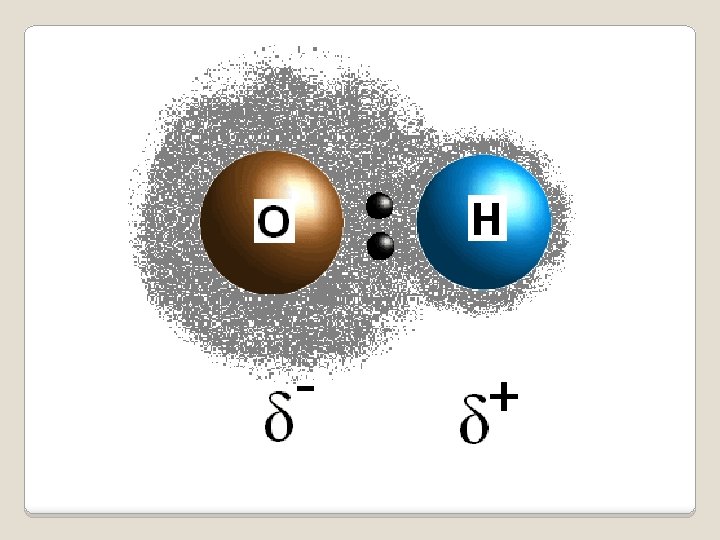

Atom with stronger electronegativity pulls electrons closer to itself Difference creates a dipole: a molecule with two poles Electronegativity

Van der Waals forces: dispersion forces and dipole forces Dispersion: weak attraction, caused by motion of electrons Dipole: polar molecules attracted to each other Hydrogen bonds: hydrogen bonded to nitrogen, oxygen, or flourine (NOF) Intermolecular Forces
- Slides: 13