Courtesy www labinitio com Naming Binary Compounds Binary

Courtesy: www. lab-initio. com
Naming Binary Compounds • Binary Compounds: compounds composed of two elements. Binary Ionic Compounds (Type I) • Binary ionic compounds contain a positive ion (cation) always written first in the formula and a negative ion (anion).

• In naming binary ionic compounds, the following rules apply: 1. The cation is always named first and the anion second. 2. A monatomic (meaning “one-atom”) cation takes its name from the name of the element. For example, Na+, is called sodium in the names of compounds containing this ion. 3. A monatomic anion is named by taking the root of the element name and adding –ide. Thus the Cl- ion is called chloride.
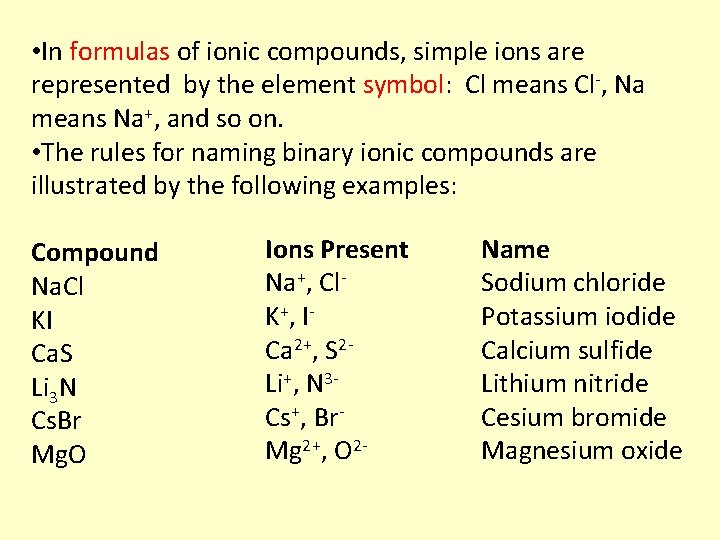
• In formulas of ionic compounds, simple ions are represented by the element symbol: Cl means Cl-, Na means Na+, and so on. • The rules for naming binary ionic compounds are illustrated by the following examples: Compound Na. Cl KI Ca. S Li 3 N Cs. Br Mg. O Ions Present Na+, Cl. K +, I Ca 2+, S 2 Li+, N 3 Cs+, Br. Mg 2+, O 2 - Name Sodium chloride Potassium iodide Calcium sulfide Lithium nitride Cesium bromide Magnesium oxide

• Formulas from names: the reverse process is also important. • Rules for Writing Formulas of Ionic Compounds 1. The positive ion is given first in the formula. 2. The subscripts in the formula must produce an electrically neutral formula unit. (Nature does require electrical neutrality. ) • Formula unit: smallest unit of an ionic compound. 3. The subscripts should be the smallest set of whole numbers possible.

Example: Write the formulas for the ionic compounds. 1. Barium sulfide The ions are Ba 2+ and S 2 -. The charges are equal but opposite, so a 1: 1 ratio will give a neutral formula unit. (2+) + (2 -) = 0 (electrical neutrality) Ba. S 2. Aluminum chloride The ions are Al 3+ and Cl- (the charge on Cl is 1 -; the one is understood). We can obtain a neutral formula unit by combining one Al 3+ with three Cl-. 1(3+) + 3(1 -) = (3+) + (3 -) = 0 Al. Cl 3

3. Aluminum oxide The ions are Al 3+ and O 2 -. In the formula there must be the same number of positive charges as negative charges for the compound to be electrically neutral. This number must be a whole-number multiple of both 3 and 2. The smallest whole number-multiple of 3 and 2 is 6, so there must be two Al 3+ and three O 2 - in the formula. 2(3+) + 3(2 -) = (6+) + (6 -) = 0 Al 2 O 3

Binary Ionic Compounds (Type II) • There are many metals that form more than one type of positive ion and thus form more than one type of ionic compound with a given anion. • For example, the compound Fe. Cl 2 contains Fe 2+ ions, and the compound Fe. Cl 3 contains Fe 3+ ions. • In this case, the charge on the metal ion must be specified in the name. • The systematic names for these two iron compounds are iron(II) chloride and iron(III) chloride, respectively. • The Roman numeral indicates the charge of the cation.

• Another system for naming these compounds is seen in older literature and still on some reagent bottles. • The ion with the higher charge has a name ending in –ic, and the one with the lower charge has a name ending in – ous. • For example, Fe 3+ is called the ferric iron, and Fe 2+ is called the ferrous ion. • The names for Fe. Cl 3 and Fe. Cl 2 are then ferric chloride and ferrous chloride, respectively.
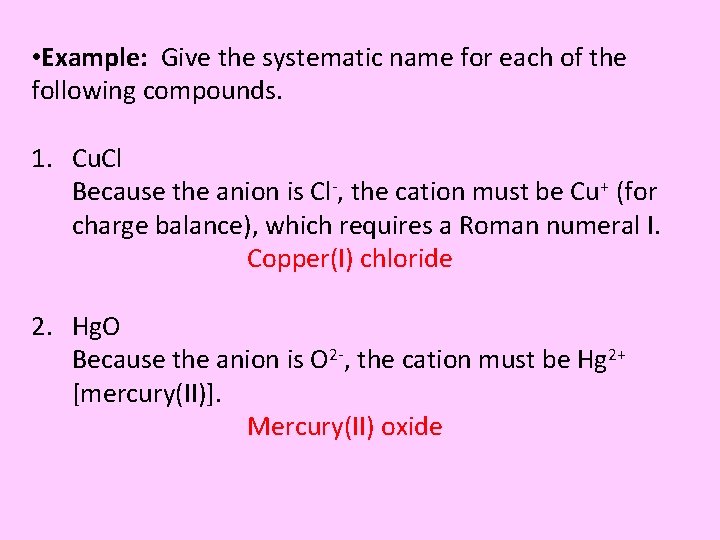
• Example: Give the systematic name for each of the following compounds. 1. Cu. Cl Because the anion is Cl-, the cation must be Cu+ (for charge balance), which requires a Roman numeral I. Copper(I) chloride 2. Hg. O Because the anion is O 2 -, the cation must be Hg 2+ [mercury(II)]. Mercury(II) oxide

3. Fe 2 O 3 The three O 2 - ions carry a total charge of 6 -, so the total positive charge must be 6+ for electrical neutrality. There are two Fe ions so 6+ divided by 2 = 3+. The Fe ion is Fe 3+ [iron(III)]. Iron(III) oxide

• Example: Given the following systematic names, write the formula for each compound. 1. Manganese(IV) oxide The manganese ion has a 4+ charge. There is only one so the total positive charge is 4+. Thus, the total negative charge must be 4 -. (4+) + (4 -) = 0 (electrical neutrality) Oxygen ions (oxide) carry a 2 - charge; two O 2 - ions (total charge 4 -) are required by the Mn 4+ ion. Mn. O 2

2. Lead(II) chloride The lead ion is Pb 2+. Chloride ion has a 1 - charge (Cl-). For electrical neutrality two Cl- ions would be required. (2+) + (2 x 1 -) = (2+) + (2 -) = 0 Pb. Cl 2

• Example: Name the following using the old nomenclature system. 1. Cr. Cl 3 There are three Cl- ions giving a total 3 - charge which must be balanced by a 3+ charge. Only one chromium ion is given in the formula so it must have a 3+ charge. Remember the ion with the higher charge has a name ending in –ic and the one with the lower charge has a name ending in –ous. Chromium forms the Cr 2+ ion and the Cr 3+ ion. Chromic chloride

2. Pb. S Sulfide ion has a 2 - charge (S 2 -) so to balance the charges the lead ion must have a 2+ charge (Pb 2+). Remember lead forms two ions: Pb 2+ and Pb 4+. Since we are using the ion with the lower charge it will have the ending –ous. Plumbous sulfide
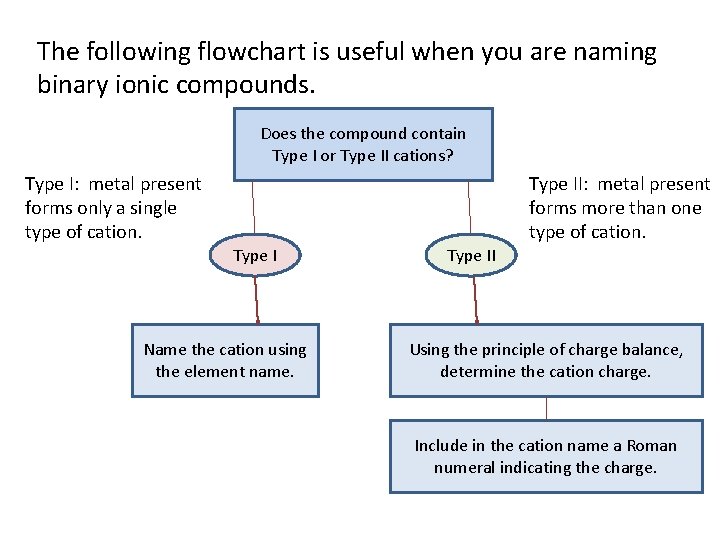
The following flowchart is useful when you are naming binary ionic compounds. Does the compound contain Type I or Type II cations? Type I: metal present forms only a single type of cation. Type II: metal present forms more than one type of cation. Type I Name the cation using the element name. Type II Using the principle of charge balance, determine the cation charge. Include in the cation name a Roman numeral indicating the charge.
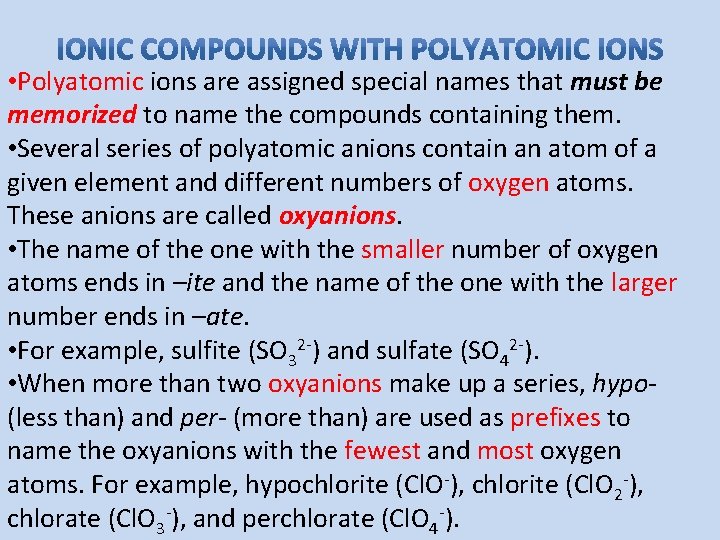
• Polyatomic ions are assigned special names that must be memorized to name the compounds containing them. • Several series of polyatomic anions contain an atom of a given element and different numbers of oxygen atoms. These anions are called oxyanions. • The name of the one with the smaller number of oxygen atoms ends in –ite and the name of the one with the larger number ends in –ate. • For example, sulfite (SO 32 -) and sulfate (SO 42 -). • When more than two oxyanions make up a series, hypo(less than) and per- (more than) are used as prefixes to name the oxyanions with the fewest and most oxygen atoms. For example, hypochlorite (Cl. O-), chlorite (Cl. O 2 -), chlorate (Cl. O 3 -), and perchlorate (Cl. O 4 -).
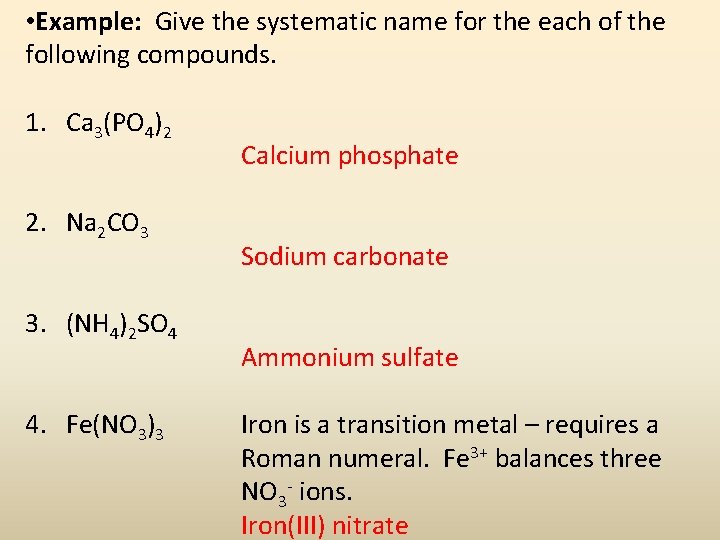
• Example: Give the systematic name for the each of the following compounds. 1. Ca 3(PO 4)2 2. Na 2 CO 3 3. (NH 4)2 SO 4 4. Fe(NO 3)3 Calcium phosphate Sodium carbonate Ammonium sulfate Iron is a transition metal – requires a Roman numeral. Fe 3+ balances three NO 3 - ions. Iron(III) nitrate

• Example: Given the following systematic names, write the formula for each compound. 1. Calcium sulfate Calcium forms an ion with a 2+ charge (Ca 2+) and the sulfate ion has a 2 - charge (SO 42 -). One Ca 2+ ion balances charge with one SO 42+ ion. Ca. SO 4 2. Iron(III) acetate The iron ion has a 3+ charge (Fe 3+) and the acetate ion has a 1 - charge (C 2 H 3 O 2 -). Three acetate ions are needed to balance charge. Fe(C 2 H 3 O 2)3 Note: the formula for the acetate is written with parentheses to show it appears three times.
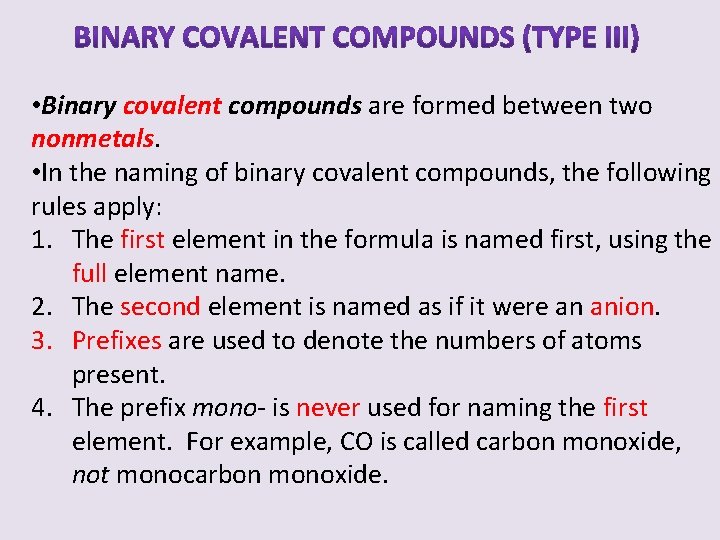
• Binary covalent compounds are formed between two nonmetals. • In the naming of binary covalent compounds, the following rules apply: 1. The first element in the formula is named first, using the full element name. 2. The second element is named as if it were an anion. 3. Prefixes are used to denote the numbers of atoms present. 4. The prefix mono- is never used for naming the first element. For example, CO is called carbon monoxide, not monocarbon monoxide.
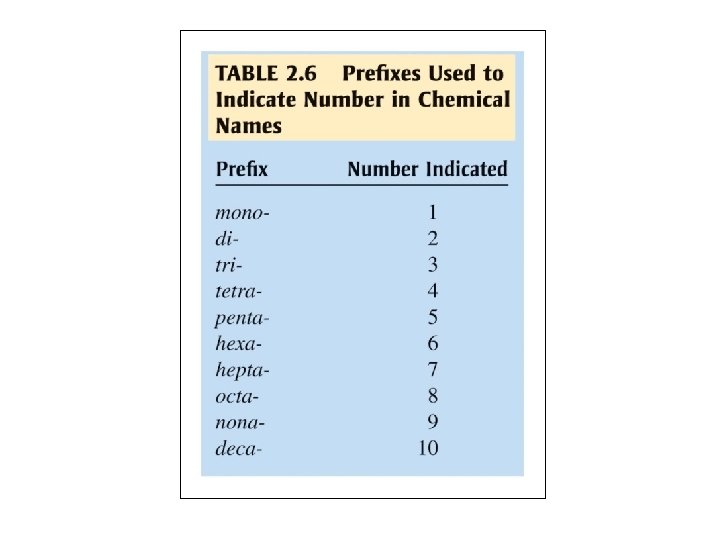
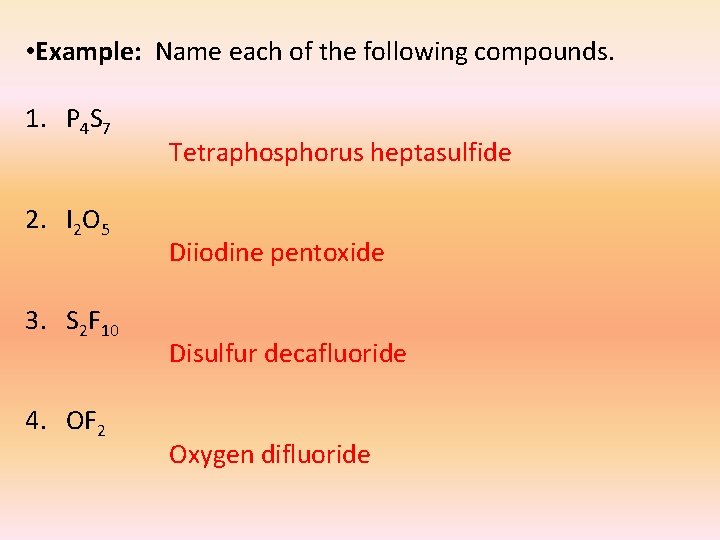
• Example: Name each of the following compounds. 1. P 4 S 7 2. I 2 O 5 3. S 2 F 10 4. OF 2 Tetraphosphorus heptasulfide Diiodine pentoxide Disulfur decafluoride Oxygen difluoride
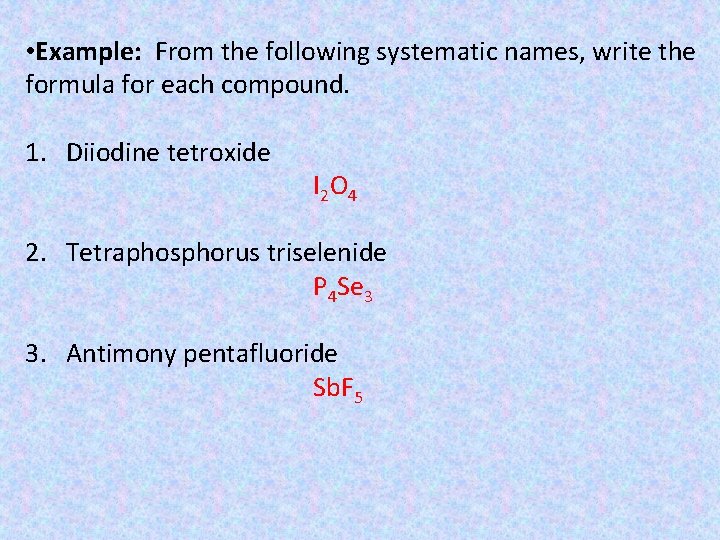
• Example: From the following systematic names, write the formula for each compound. 1. Diiodine tetroxide I 2 O 4 2. Tetraphosphorus triselenide P 4 Se 3 3. Antimony pentafluoride Sb. F 5
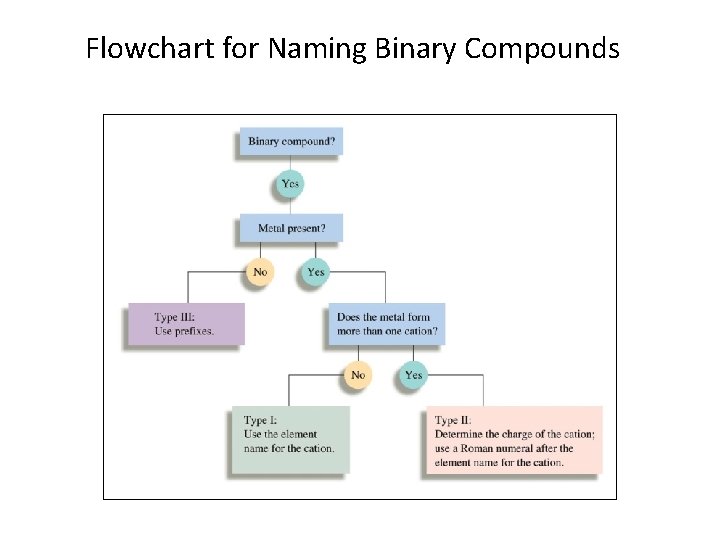
Flowchart for Naming Binary Compounds
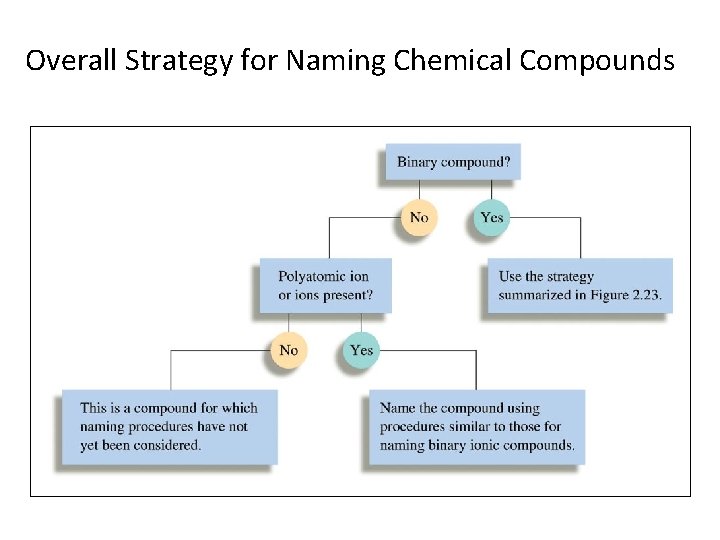
Overall Strategy for Naming Chemical Compounds
Acids • An acid can be viewed as a molecule with one or more H+ ions attached to an anion. • The rules for naming acids depend on whether the anion contains oxygen. • If the anion does not contain oxygen, the acid is named with the prefix hydro- and the suffix –ic. • For example, when gaseous HCl is dissolved in water, it forms hydrochloric acid.

• When the anion contains oxygen, the acidic name is formed from the root name of the anion with a suffix of –ic or –ous, depending on the name of the anion. 1. If the anion name ends in –ate, the suffix –ic is added to the root name. For example, H 2 SO 4 contains the sulfate anion (SO 42 -) and is called sulfuric acid. 2. If the anion has an –ite ending, the –ite is replaced by –ous. For example, H 2 SO 3, which contains sulfite (SO 32 -), is named sulfurous acid.
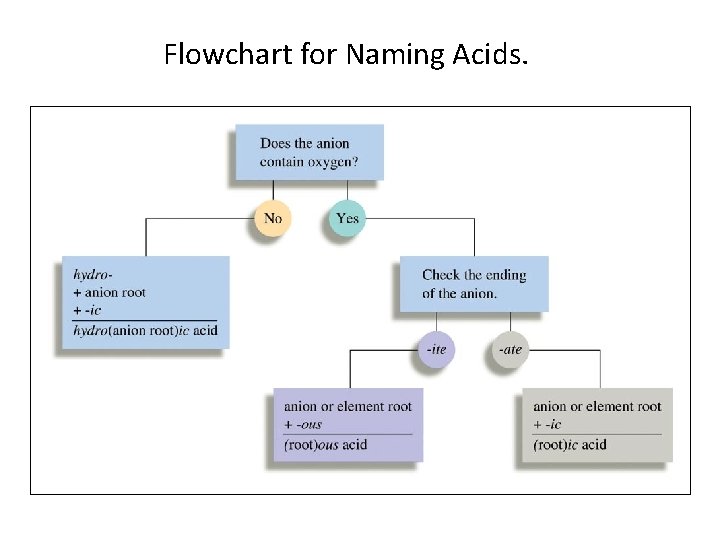
Flowchart for Naming Acids.

• Example: name the following acids. 1. HI(g) dissolved in water The anion does not contain oxygen so the prefix hydroand the suffix –ic is added. Hydroiodic acid 2. HCl. O 4 The anion contains oxygen and is called perchlorate so the suffix –ic is added to the name. Perchloric acid
- Slides: 29