Course Name Psychrometry Basics Prof A D Kale

Course Name: Psychrometry Basics Prof. A. D. Kale Vishwakarma Institute of Information Technology, Pune

Dry & Atmospheric Air • Air is a mixture of N 2, O 2 and small amount of other gasses. • Atmospheric air: Air in the atmosphere normally contains some water vapor (a moisture) and is referred to as atmospheric air. • Dry air: Air that contains no water vapor is called dry air. • The temperature of air in air-conditioning application ranges between -10 OC to 50 OC. 2
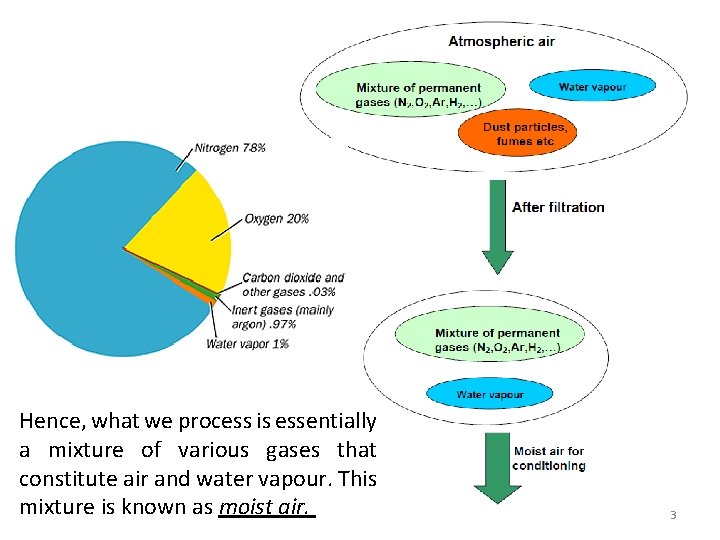
Hence, what we process is essentially a mixture of various gases that constitute air and water vapour. This mixture is known as moist air. 3
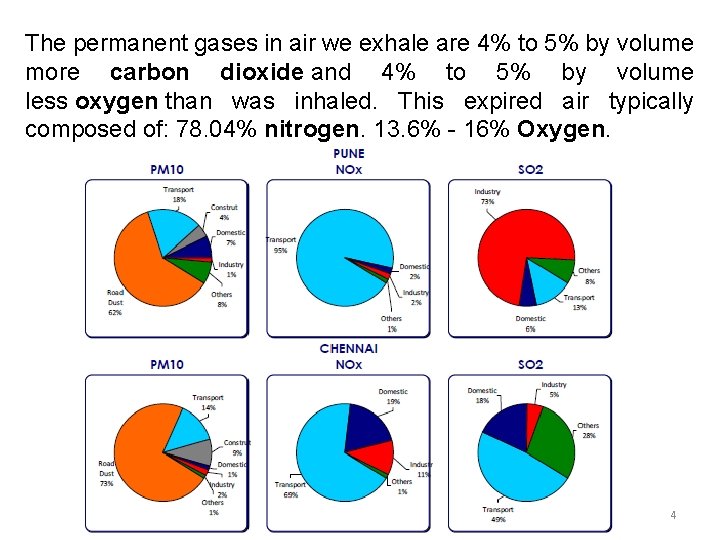
The permanent gases in air we exhale are 4% to 5% by volume more carbon dioxide and 4% to 5% by volume less oxygen than was inhaled. This expired air typically composed of: 78. 04% nitrogen. 13. 6% - 16% Oxygen. 4
Psychrometrics is the science of moist air properties and processes, which is used to illustrate and analyze air-conditioning cycles. It translates the knowledge of heating or cooling loads (which are in k. W or tons) into volume flow rates (in m 3/s or cfm) for the air to be circulated into the duct system. Psychrometry is the study of the properties of mixtures of air and water vapour. Copyright 2002, American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc. (www. ashrae. org). Reprinted by permission from ASHRAE Journal, July 2002. 5
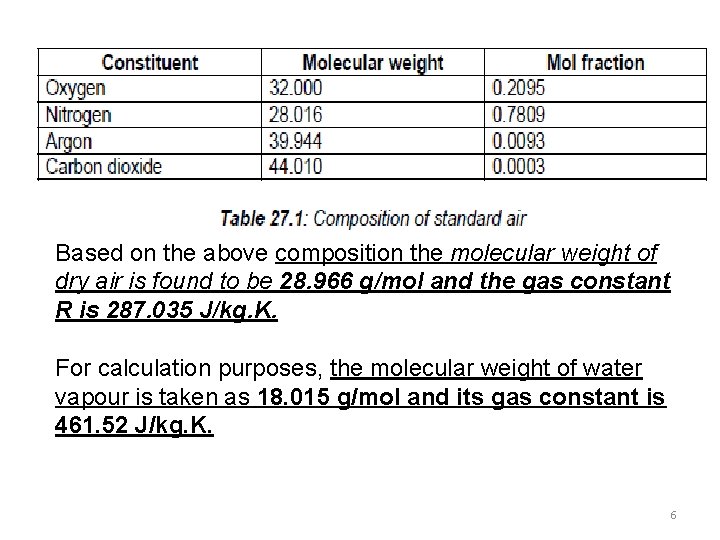
Based on the above composition the molecular weight of dry air is found to be 28. 966 g/mol and the gas constant R is 287. 035 J/kg. K. For calculation purposes, the molecular weight of water vapour is taken as 18. 015 g/mol and its gas constant is 461. 52 J/kg. K. 6

At a given temperature and pressure; the dry air can only hold a certain maximum amount of moisture. When the moisture content is maximum, then the air is known as saturated air, which is established by a neutral equilibrium between the moist air and the liquid or solid phases of water. 7

Saturated Air: • There is a limit on the amount of vapor the air can hold at a given temperature. • Air that is holding as much moisture as it can at a given temperature is called saturated air. • Any further moisture introduced into saturated air will condense. • When air and saturated water vapor occupy the same volume, we say air is saturated. In reality only the water vapor in the given air is saturated. 8

• The atmospheric air can be treated as an ideal -gas mixture whose pressure is the sum of the partial pressure of dry air Pa and that of water vapor Pv. • Vapor Pressure: The partial pressure of water vapor is usually referred to as the vapor pressure. It is the pressure water vapor would exert if it existed alone at the temperature and volume of atmospheric air. 9
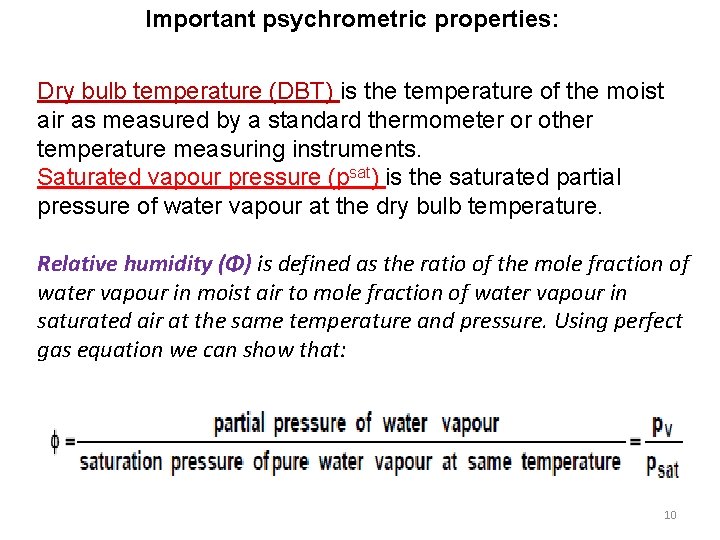
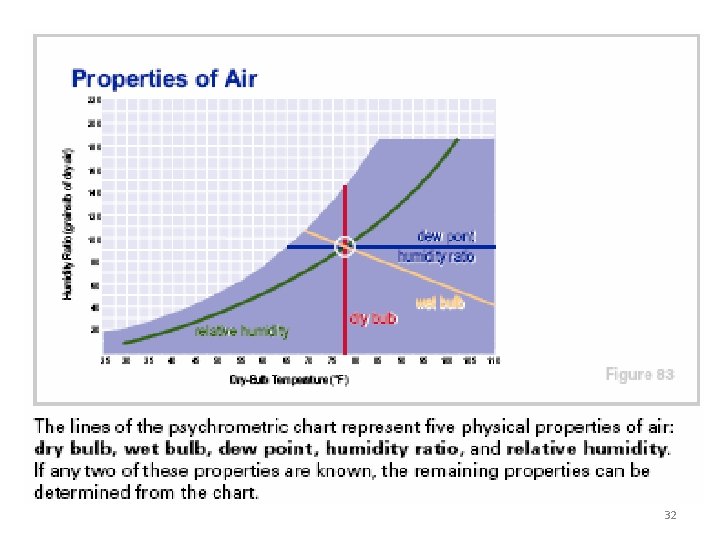
Important psychrometric properties: Dry bulb temperature (DBT) is the temperature of the moist air as measured by a standard thermometer or other temperature measuring instruments. Saturated vapour pressure (psat) is the saturated partial pressure of water vapour at the dry bulb temperature. Relative humidity (Φ) is defined as the ratio of the mole fraction of water vapour in moist air to mole fraction of water vapour in saturated air at the same temperature and pressure. Using perfect gas equation we can show that: 10
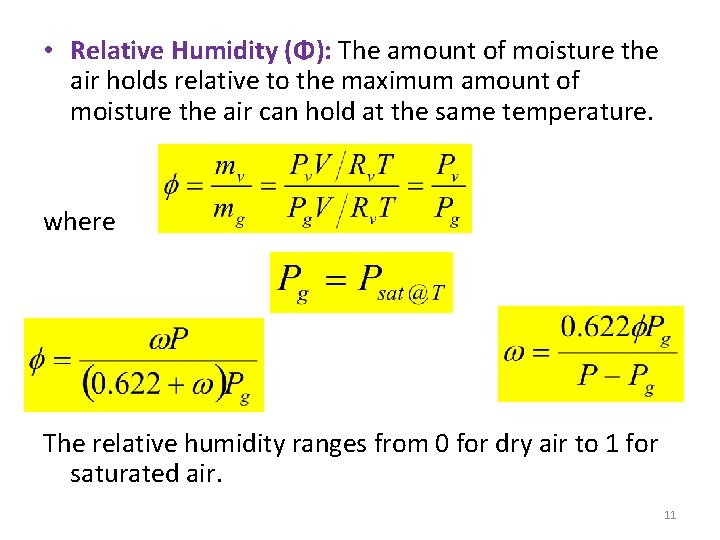
• Relative Humidity (Φ): The amount of moisture the air holds relative to the maximum amount of moisture the air can hold at the same temperature. where The relative humidity ranges from 0 for dry air to 1 for saturated air. 11
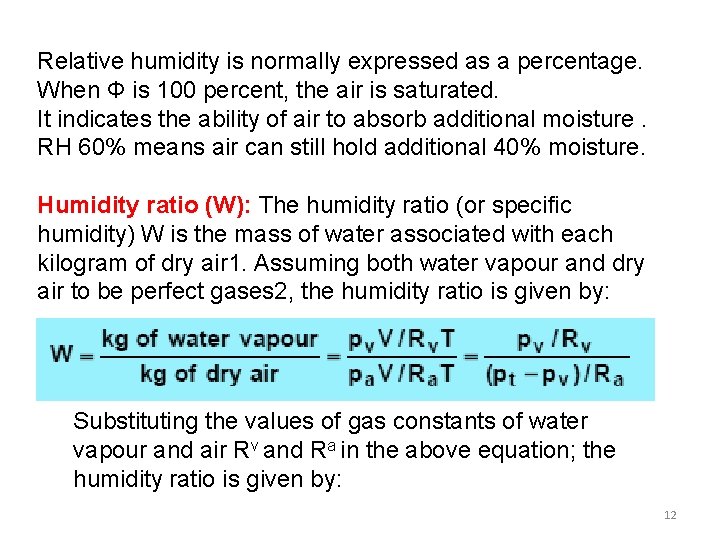
Relative humidity is normally expressed as a percentage. When Φ is 100 percent, the air is saturated. It indicates the ability of air to absorb additional moisture. RH 60% means air can still hold additional 40% moisture. Humidity ratio (W): The humidity ratio (or specific humidity) W is the mass of water associated with each kilogram of dry air 1. Assuming both water vapour and dry air to be perfect gases 2, the humidity ratio is given by: Substituting the values of gas constants of water vapour and air Rv and Ra in the above equation; the humidity ratio is given by: 12
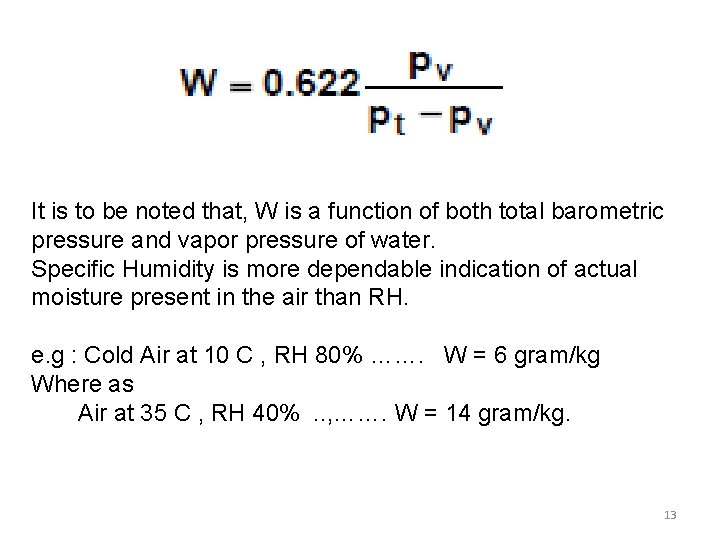
It is to be noted that, W is a function of both total barometric pressure and vapor pressure of water. Specific Humidity is more dependable indication of actual moisture present in the air than RH. e. g : Cold Air at 10 C , RH 80% ……. W = 6 gram/kg Where as Air at 35 C , RH 40% . . , ……. W = 14 gram/kg. 13
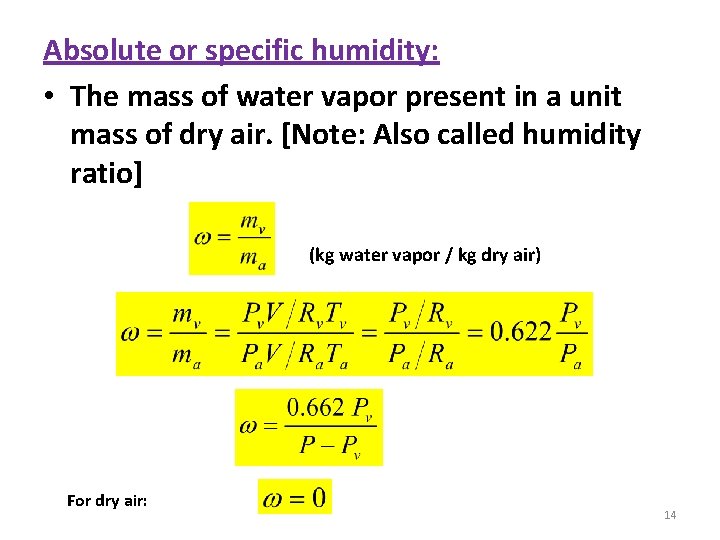
Absolute or specific humidity: • The mass of water vapor present in a unit mass of dry air. [Note: Also called humidity ratio] (kg water vapor / kg dry air) For dry air: 14
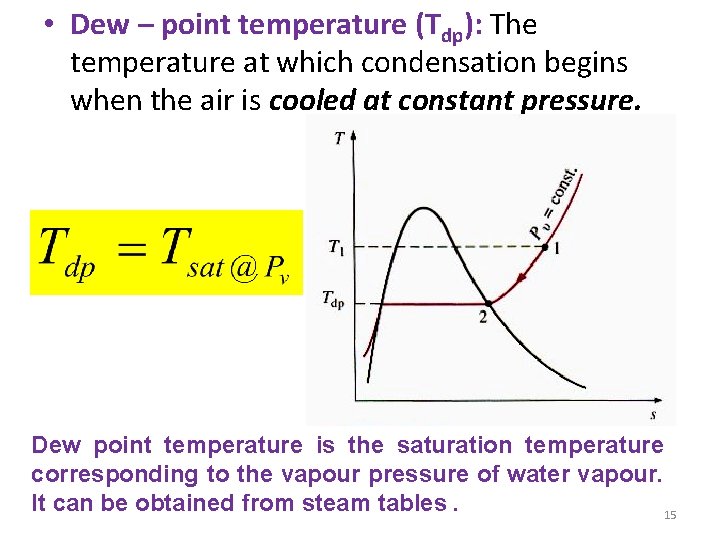
• Dew – point temperature (Tdp): The temperature at which condensation begins when the air is cooled at constant pressure. Dew point temperature is the saturation temperature corresponding to the vapour pressure of water vapour. It can be obtained from steam tables. 15

• When the temperature of a cold drink is below the dew-point temperature of the surrounding air, it ‘‘sweats. ” 16
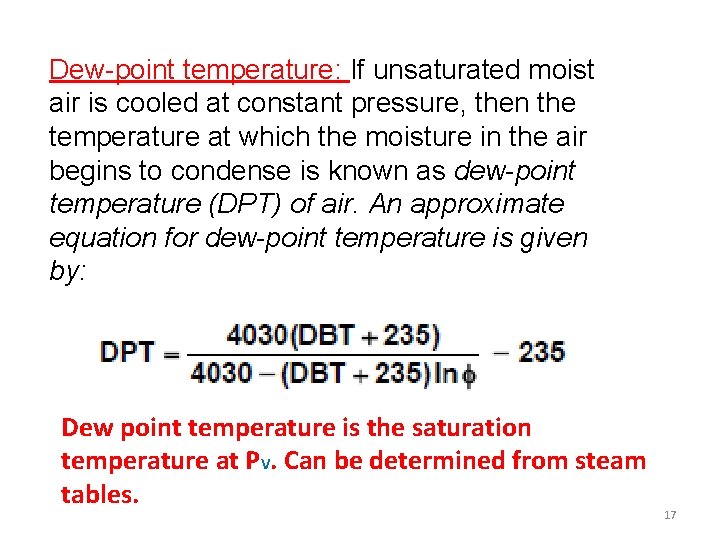
Dew-point temperature: If unsaturated moist air is cooled at constant pressure, then the temperature at which the moisture in the air begins to condense is known as dew-point temperature (DPT) of air. An approximate equation for dew-point temperature is given by: Dew point temperature is the saturation temperature at PV. Can be determined from steam tables. 17

Enthalpy: The enthalpy of moist air is the sum of the enthalpy of the dry air and the enthalpy of the water vapour. Enthalpy values are always based on some reference value. For moist air, the enthalpy of dry air is given a zero value at 0 o. C, and for water vapour the enthalpy of saturated water is taken as zero at 0 o. C. The enthalpy of moist air is given by: Or Give Other Formulae t = Dry-bulb temperature of air-vapor mixture, o. C W = Humidity ratio, kg of water vapor/kg of dry air The unit of h is k. J/kg of dry air. 18
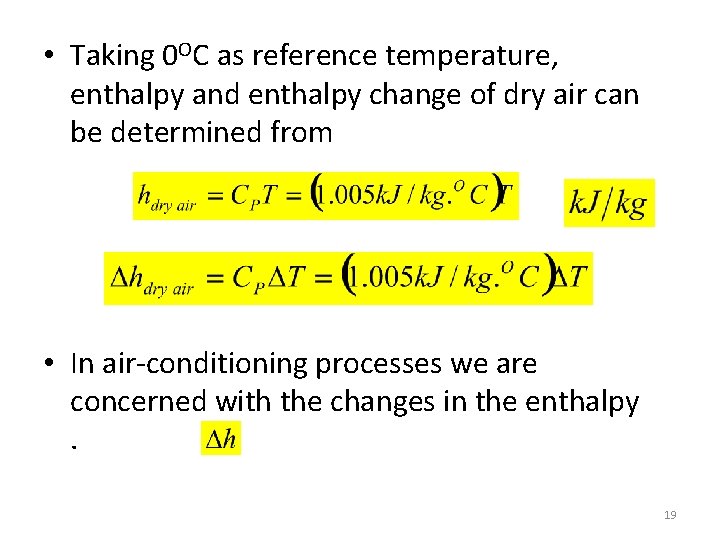
• Taking 0 OC as reference temperature, enthalpy and enthalpy change of dry air can be determined from • In air-conditioning processes we are concerned with the changes in the enthalpy. 19
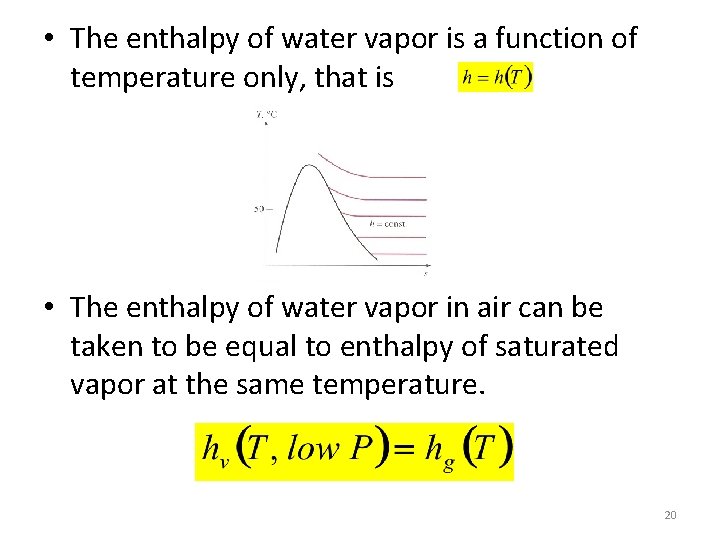
• The enthalpy of water vapor is a function of temperature only, that is • The enthalpy of water vapor in air can be taken to be equal to enthalpy of saturated vapor at the same temperature. 20
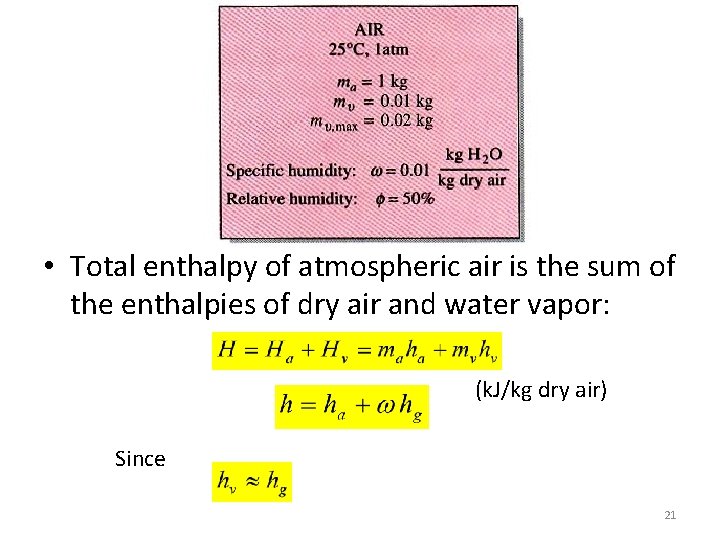
• Total enthalpy of atmospheric air is the sum of the enthalpies of dry air and water vapor: (k. J/kg dry air) Since 21
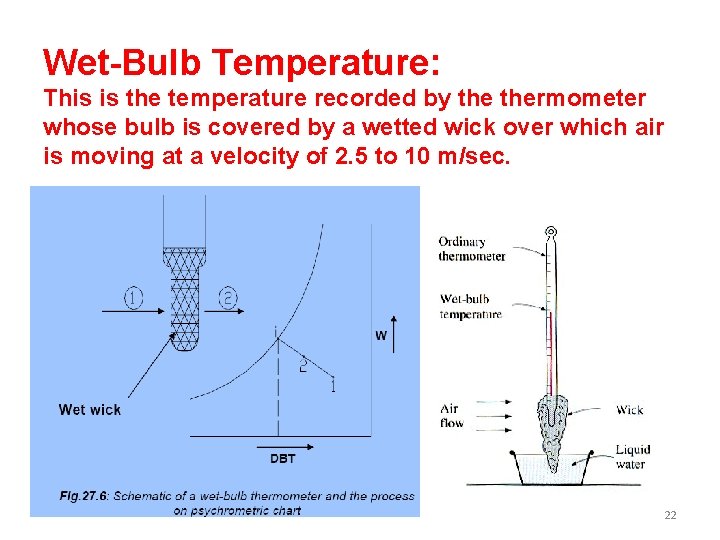
Wet-Bulb Temperature: This is the temperature recorded by thermometer whose bulb is covered by a wetted wick over which air is moving at a velocity of 2. 5 to 10 m/sec. 22
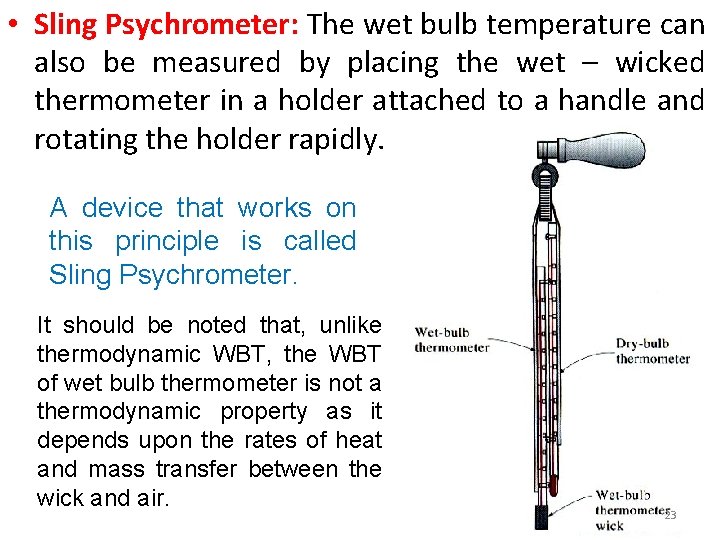
• Sling Psychrometer: The wet bulb temperature can also be measured by placing the wet – wicked thermometer in a holder attached to a handle and rotating the holder rapidly. A device that works on this principle is called Sling Psychrometer. It should be noted that, unlike thermodynamic WBT, the WBT of wet bulb thermometer is not a thermodynamic property as it depends upon the rates of heat and mass transfer between the wick and air. 23
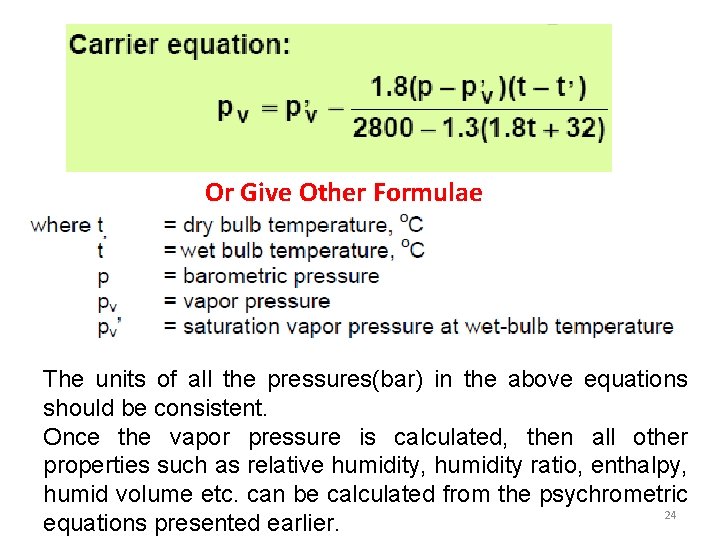
Or Give Other Formulae The units of all the pressures(bar) in the above equations should be consistent. Once the vapor pressure is calculated, then all other properties such as relative humidity, humidity ratio, enthalpy, humid volume etc. can be calculated from the psychrometric 24 equations presented earlier.
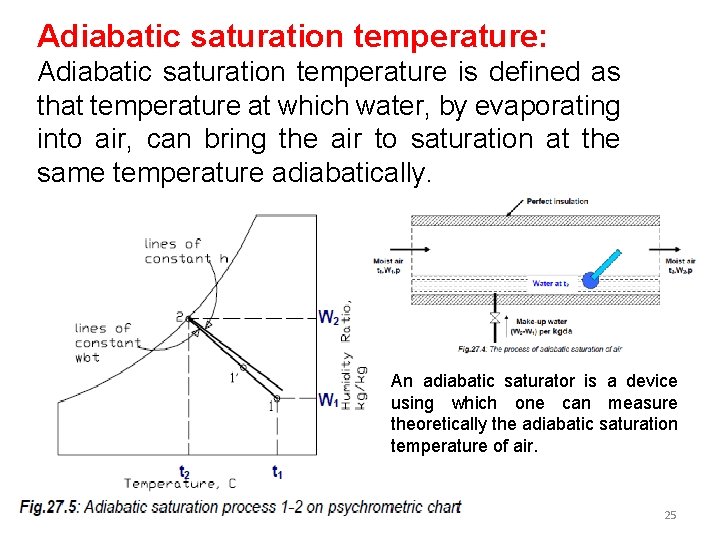
Adiabatic saturation temperature: Adiabatic saturation temperature is defined as that temperature at which water, by evaporating into air, can bring the air to saturation at the same temperature adiabatically. An adiabatic saturator is a device using which one can measure theoretically the adiabatic saturation temperature of air. 25
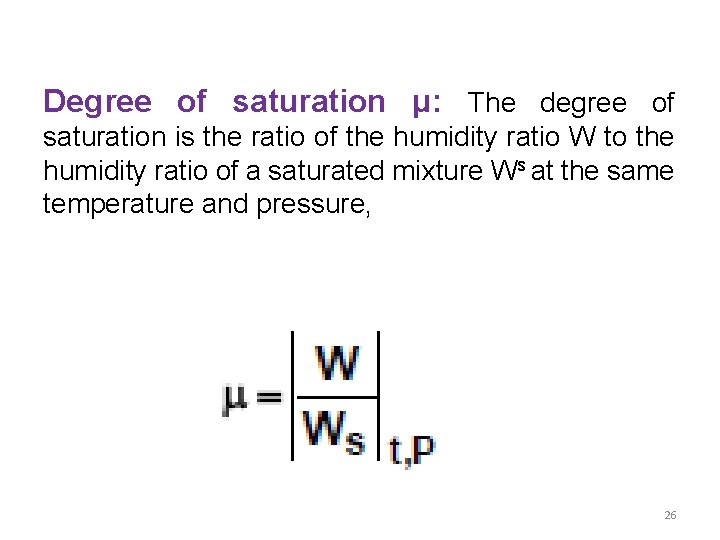
Degree of saturation μ: The degree of saturation is the ratio of the humidity ratio W to the humidity ratio of a saturated mixture Ws at the same temperature and pressure, 26

Numerical Types: 1) With only standard psychrometric formulae 2) By using Psychrometric Chart 27
Psychrometric Chart : • The properties of atmospheric air at a specified total pressure are presented in the form of easily readable charts called Psycrometric Chart. • The dry – bulb temperature are shown on the horizontal axis. • The specific humidity is shown on the vertical axis. 28
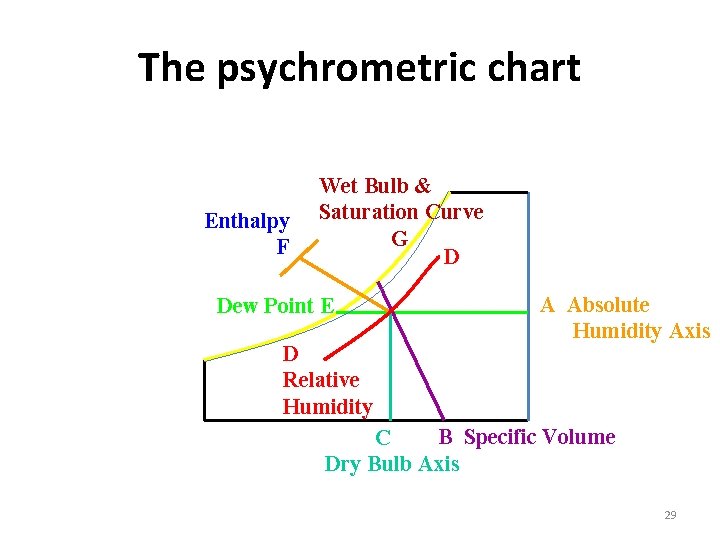
The psychrometric chart Enthalpy F Wet Bulb & Saturation Curve G D Dew Point E D Relative Humidity A Absolute Humidity Axis B Specific Volume C Dry Bulb Axis 29
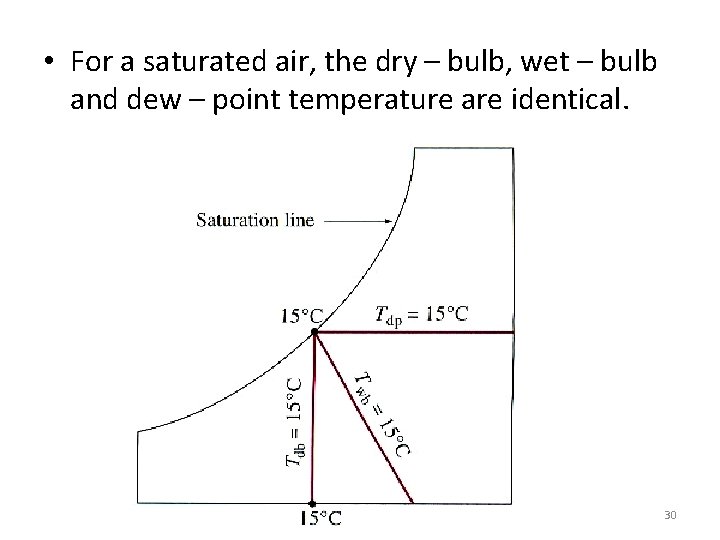
• For a saturated air, the dry – bulb, wet – bulb and dew – point temperature are identical. 30
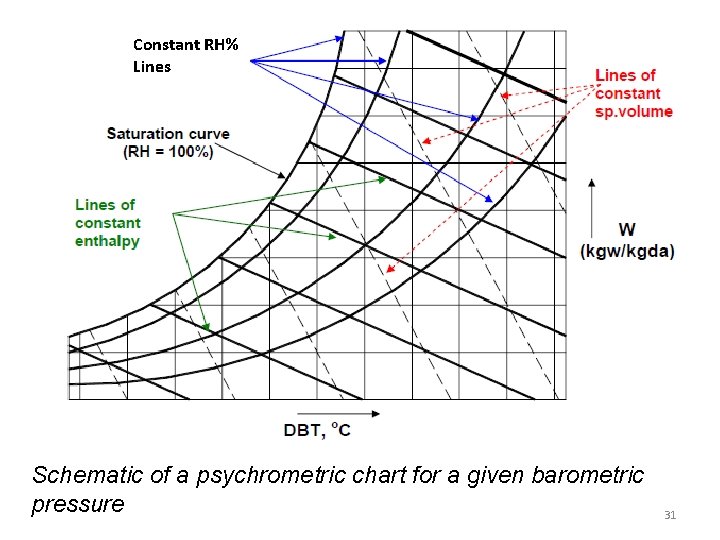
Constant RH% Lines Schematic of a psychrometric chart for a given barometric pressure 31
32
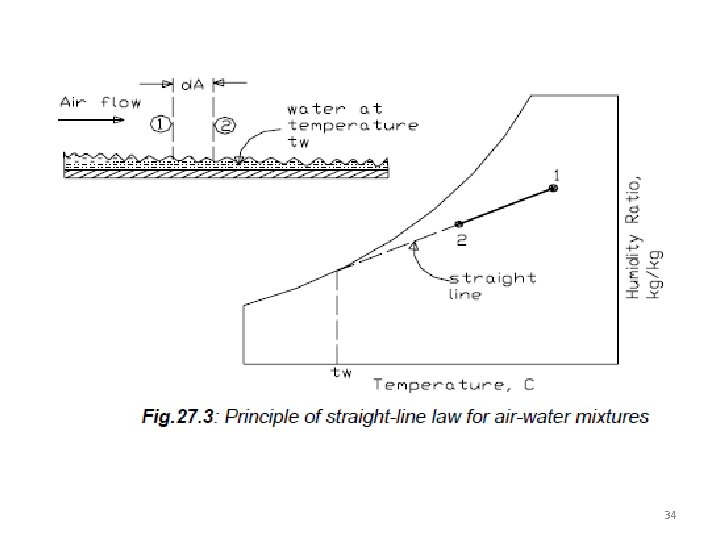
Combined heat and mass transfer; the straight line law The straight line law states that “when air is transferring heat and mass (water) to or from a wetted surface, the condition of air shown on a psychrometric chart drives towards the saturation line at the temperature of the wetted surface”. For example, as shown In figure when warm air passes over a wetted surface its temperature drops from 1 to 2. Also, since the vapor pressure of air at 1 is greater than the saturated vapor pressure at tw, there will be moisture transfer from air to water, i. e. , the warm air in contact with cold wetted surface cools and dehumidifies. According to the straight line law, the final condition of air (i. e. , 2) lies on a straight line joining 1 with tw on the saturation line. This is due to the value of unity of the Lewis number, 33
34
- Slides: 34