Course medicinal plants Muhammad Qasim Hayat Alkaloids group


































![Sources [1] Dioscoreaceae (yam family) ▪ Dioscorea genus – dicots – vines ▪ sweet Sources [1] Dioscoreaceae (yam family) ▪ Dioscorea genus – dicots – vines ▪ sweet](https://slidetodoc.com/presentation_image_h/a4c50c3cf89e387e94c667513caefcb6/image-51.jpg)

























- Slides: 81

Course: medicinal plants Muhammad Qasim Hayat

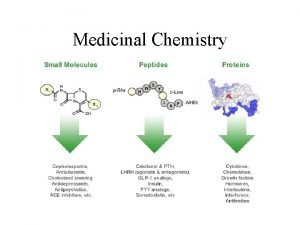
Alkaloids ▪ group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. ▪ also includes some related compounds with neutral and even weakly acidic properties ▪ Low molecular weight Nitrogenous compounds ▪ Mostly metabolic by-products derived from amino acids. ▪ A. k. a. secondary metabolites ▪ produced by a large variety of organisms, including bacteria, fungi, plants, and animals.

Chemical structure ▪ Alkaloids contain; ▪ carbon, ▪ hydrogen ▪ nitrogen ▪ In addition, may also contain ▪ oxygen, ▪ sulfur and ▪ rarely other elements such as chlorine, bromine, and phosphorus. ▪ contain one or more phenolic or indole rings, usually with a nitrogen atom in the ring ▪ position of the nitrogen atom in the carbon ring varies with different alkaloids ▪ it is the precise position of the nitrogen atom that effects the properties of these alkaloids.


▪ The boundary between alkaloids and other nitrogen-containing natural compounds is not clear -cut. ▪ Compounds like amino acid peptides, proteins, nucleotides, nucleic acid, amines, and antibiotics are usually not called alkaloids. ▪ alkaloids are at times called a special case of amines.

Chemical properties ▪ Bitter and alkaline ▪ Oxygen-containing alkaloids; ▪ usually colorless crystals at ambient conditions. ▪ Oxygen-free alkaloids; ▪ are typically volatile, colorless, oily liquids. ▪ Some alkaloids are colored eg: sanguinarine (orange). ▪ Alkaloids are poorly soluble in water but readily dissolve in organic solvents, such as diethyl ether, chloroform ▪ Exceptions such as caffeine dissolves well in boiling water. [ ▪ With acids, alkaloids form salts of various strengths. ▪ Those salts are usually soluble in water and alcohol and poorly soluble in most organic solvents.

Identification tests: Qualitative ▪ Mayor’s test ▪ 1 ml of extract + 1 ml of Mayer’s reagent (potassium mercuric iodide solution). Whitish or cream colored precipitate indicates the presence of alkaloids. ▪ Wagner’s test ▪ 1 ml of extract + 2 ml of Wagner’s reagent (iodine in potassium iodide). Reddish brown colored precipitate indicates the presence of alkaloids ▪ Dragendorff’s test ▪ 1 ml of extract + 1 ml of Dragendroff’s reagent (potassium bismuth iodide solution). An orange-red precipitate indicates the presence of alkaloids. ▪ Hager’s test ▪ 1 ml of extract + 3 ml of Hager’s reagent (saturated aqueous solution of picric acid). Yellow colored precipitate indicates the presence of alkaloids

Identification tests : Quantitative ▪ Spectrophotometric determination ▪ HPLC (high performance liquid chromatography)

Extraction ▪ Because of the structural diversity of alkaloids, there is no single method of their extraction from natural raw materials. ▪ Most methods exploit the solubility of most alkaloids in organic solvents but not in water, and the opposite tendency of their salts. ▪ Many alkaloids can be purified from crude extracts by acid-base extraction.

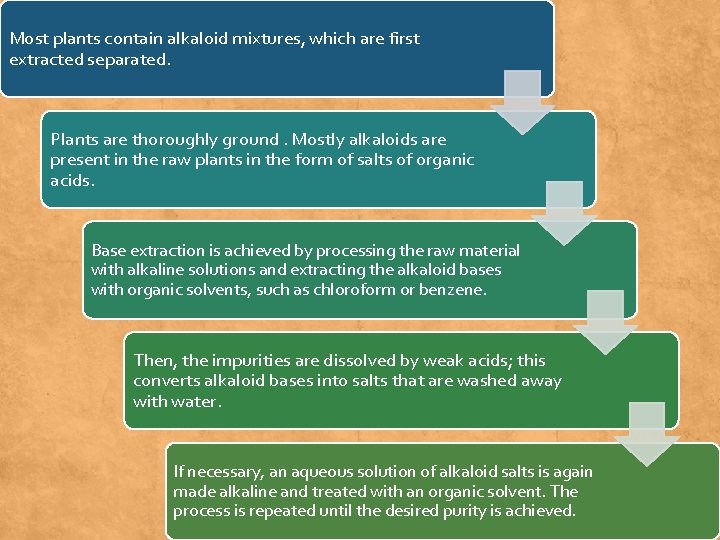
Most plants contain alkaloid mixtures, which are first extracted separated. Plants are thoroughly ground. Mostly alkaloids are present in the raw plants in the form of salts of organic acids. Base extraction is achieved by processing the raw material with alkaline solutions and extracting the alkaloid bases with organic solvents, such as chloroform or benzene. Then, the impurities are dissolved by weak acids; this converts alkaloid bases into salts that are washed away with water. If necessary, an aqueous solution of alkaloid salts is again made alkaline and treated with an organic solvent. The process is repeated until the desired purity is achieved.

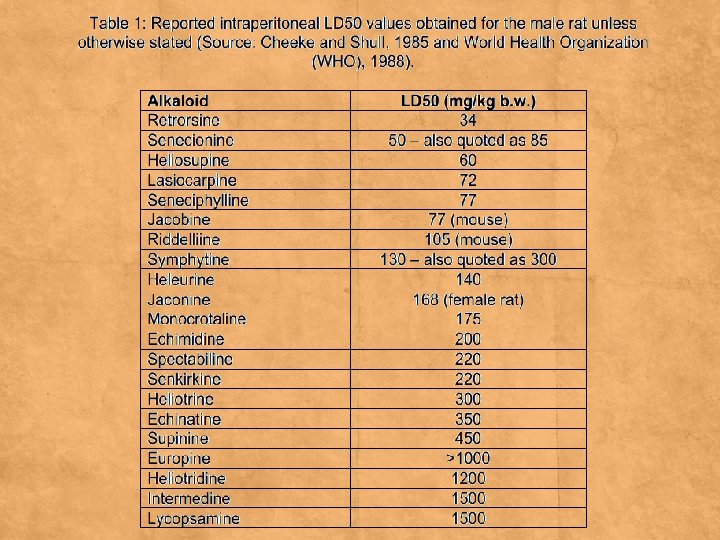
Toxicology ▪ Many alkaloids are toxic to other organisms. ▪ Example ▪ Pyrrolizidine alkaloids (PAs) are a large group of natural toxins produced by plants, several of which are known to be highly hepatotoxic and have been shown to be carcinogenic in rats. ▪ They have been associated with a number of livestock diseases and with cases of human poisoning following consumption of herbal remedies or after contamination of staple foods. ▪ Example plants in ▪ Boraginaceae, Asteraceae, Orchidaceae families


Medicinal properties ▪ Alkaloid-containing plants have been used by humans since ancient times for therapeutic and recreational purposes. ▪ For example, A Chinese book on houseplants written in 1 st– 3 rd centuries BC mentioned a medical use of opium poppies. ▪ They often have pharmacological effects and are used as medications ▪ Some alkaloids have remarkable structural similarities with neurotransmitters in the central nervous system of humans, including dopamine, serotonin and acetylcholine. ▪ The amazing effect of these alkaloids on humans has led to the development of powerful pain-killer medications.

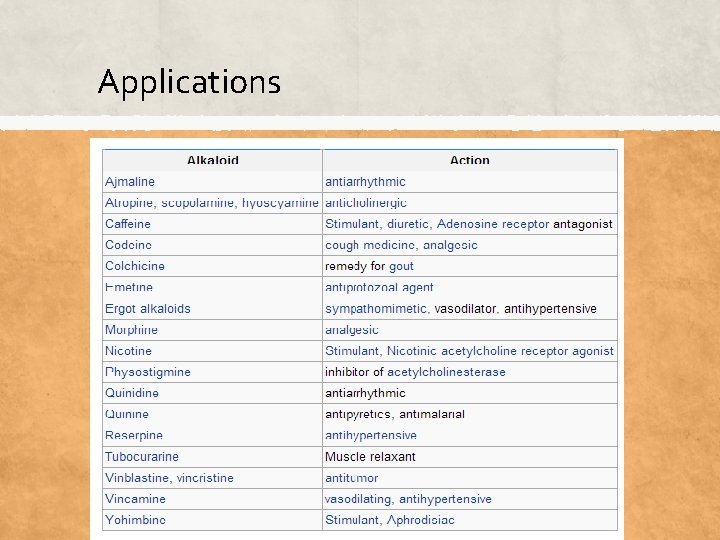
Applications

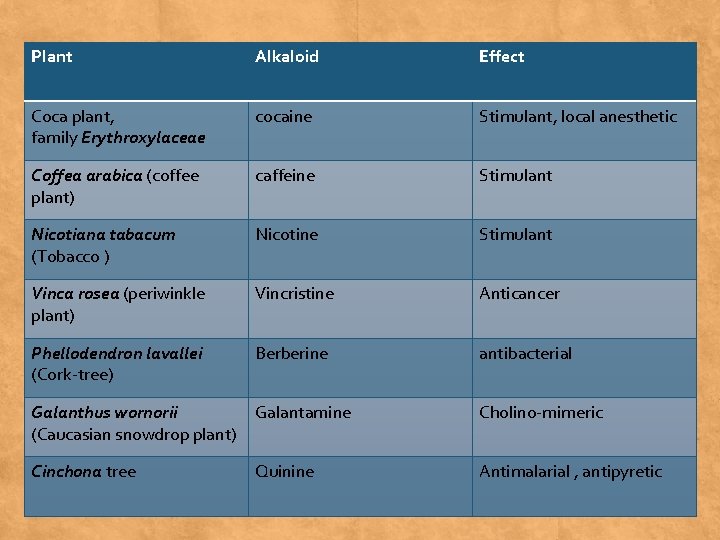
Plant Alkaloid Effect Coca plant, family Erythroxylaceae cocaine Stimulant, local anesthetic Coffea arabica (coffee plant) caffeine Stimulant Nicotiana tabacum (Tobacco ) Nicotine Stimulant Vinca rosea (periwinkle plant) Vincristine Anticancer Phellodendron lavallei (Cork-tree) Berberine antibacterial Galanthus wornorii Galantamine (Caucasian snowdrop plant) Cholino-mimeric Cinchona tree Antimalarial , antipyretic Quinine


Fats AND OILS Muhammad Qasim Hayat

INTRODUCTION • Fats and Oils belong to a group of biological subtance called Lipids. lipids are biological chemicals do not dissolve in water FUNCTIONs 1. Regulatory messenger 2. Structural component of membrane 3. Energy store house How fats differ from oils? Fats differ from oils in that, they are solid at room temperature while oils are liquid they have same molecular structure

Molecular structure of fats and oils. They are also called triglycerides. one of the reaction of triglycerides is is hydrolysis of ester groups.

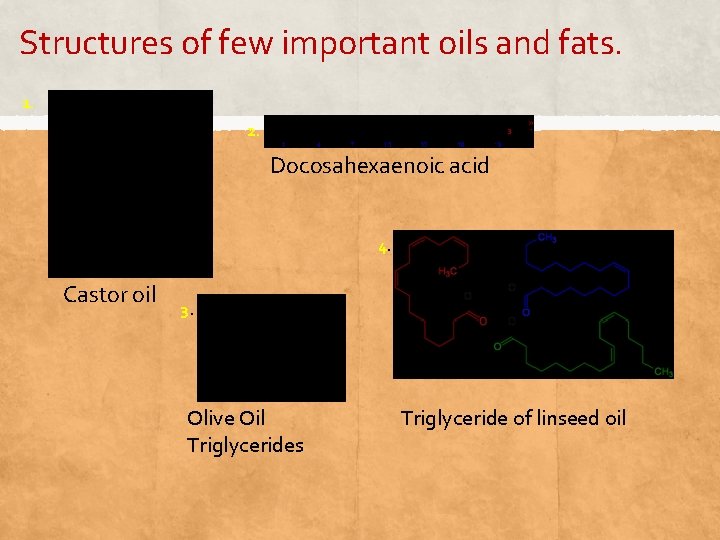
Structures of few important oils and fats. 1. 2. Docosahexaenoic acid 4. Castor oil 3. Olive Oil Triglycerides Triglyceride of linseed oil

Medicinal properties of fats and oils Fats and oils have high medicinal value • They are high energy foods, providing about 9 calories per gram of fat. • They are needed for your brain and nervous system, for energy production and for making most of the body’s vital hormones • two omega-3 essential fatty acids needed for development of the nervous system. • coconut oil, along with coconut milk and coconut water, is highly praised today due to its high content of lauric acid, a natural infection fighter. • MCT or medium chain triglygcerides appear to assist energy production in some diseases such as Alzheimer’s disease omega-3 fatty acids which are also sometimes called essential fatty acids. They are critical chemicals in our body that can help reduce inflammation, are needed for cell membranes, for nervous system development, and for many other functions in the body.

• Cholesterol is an essential fat compound manufactured in our livers that is needed to make all of the sex hormones and steroid hormones. It is mainly made in our bodies. • Symptoms of omega-3 deficiency include rashes, dry skin, boils, joint pain, irritability, mental problems and much more. • Fats and oils coat the nerves with myelin, an important fatty substance that is needed to conduct nerve impulses properly. • Castor oil is cathartic it is also used as an emollient. • Shark liver oil is used in joint pain and with Vit-D used in burns and sun burns. • Linseed oil is used in scabies and skin diseases. • Olive oil is used as laxative.

Chemical properties of fats • Fats and oils can be classified according to their degree of unsaturation as measured by their ability to absorb iodine at the double bonds. • Fats have relatively low iodine values and consist of glycerides containing high percentages of such saturated acids as lauric, myristic, and palmitic. • ; milk fats are usually characterized by the presence of shortchain carboxylic acids (butyric, caproic, and caprylic) • Fats and oils are hydrolized readily. This property is used extensively in the manufacture of soaps and in the preparation of fatty acids for industrial applications.

Toxicity of fats and oils. ▪ When an oil is saturated, that means that the molecule has all the hydrogen atoms it can hold. Unsaturation means that some hydrogen atoms have been removed, and this opens the structure of the molecule in a way that makes it susceptible to attack by free radicals. ▪ Free radicals are reactive molecular fragments that occur even in healthy cells, and can damage the cell. When unsaturated oils are exposed to free radicals they can create chain reactions of free radicals that spread the damage in the cell, and contribute to the cell's aging. ▪ Rancidity of oils occurs when they are exposed to oxygen, in the body just as in the bottle. Harmful free radicals are formed, and oxygen is used up.

Identification tests for oil and fats used 1. Quality evaluation of oils and fats ▪ Transparency ▪ Determination of impurity ▪ Color ▪ Determination of acid ▪ Odour ▪ Taste ▪ Relative density value ▪ Determination of phospholipids ▪ Refractive Index ▪ Determination of soap ▪ Smoke point ▪ Determination of ▪ Melting point ▪ Freezing point saponification value ▪. Determination of

2. QUANTITATIVE CHEMICAL TESTS ØPhysical Constants ØChemical Constants ▪ Specific Gravity ▪ Acid Values ▪ Melting / Congealing ▪ Saponification Value Point ▪ Refractive Index ▪ Viscosity ▪ Optical Rotation ▪ Ester Value ▪ Iodine Value ▪ Unsaponifiable Matter ▪ Acetyl value

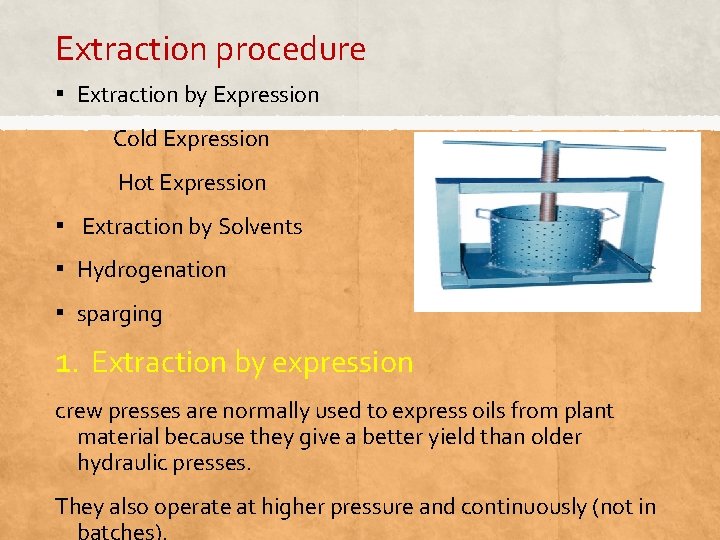
Extraction procedure ▪ Extraction by Expression Cold Expression Hot Expression ▪ Extraction by Solvents ▪ Hydrogenation ▪ sparging 1. Extraction by expression crew presses are normally used to express oils from plant material because they give a better yield than older hydraulic presses. They also operate at higher pressure and continuously (not in batches).

• EXTRACTION BY EXPRESSION ▪ COLD EXPRESSION ▪ HOT EXPRESSION Oils for medicinal uses are extracted at room temperature. Only a portion of the oil is obtained. The residue left after cold expression is broken down and treated with steam. This causes the remaining oil cells to rupture.

2. EXTRACTION BY SOLVENT This type of method is used only for technical oils (not medicinal oils). Seeds used: Intact or partially extracted by expression. Solvent: Normally hexane (BP: 65 ºC)

3. Hydrogenation ▪ Oils may be partially hydrogenated to produce various ingredient oils. Lightly hydrogenated oils have very similar physical characteristics to regular soy oil, but are more resistant to becoming rancid. Margarine oils need to be mostly solid at 32 °C (90 °F) so that the margarine does not melt in warm rooms, 4. Sparging ▪ In the processing of edible oils, the oil is heated under vacuum to near the smoke point, and water is introduced at the bottom of the oil. The water immediately is converted to steam, which bubbles through the oil, carrying with it any chemicals which are water-soluble. The steam sparging removes impurities that can impart unwanted flavors and odors to the oil.

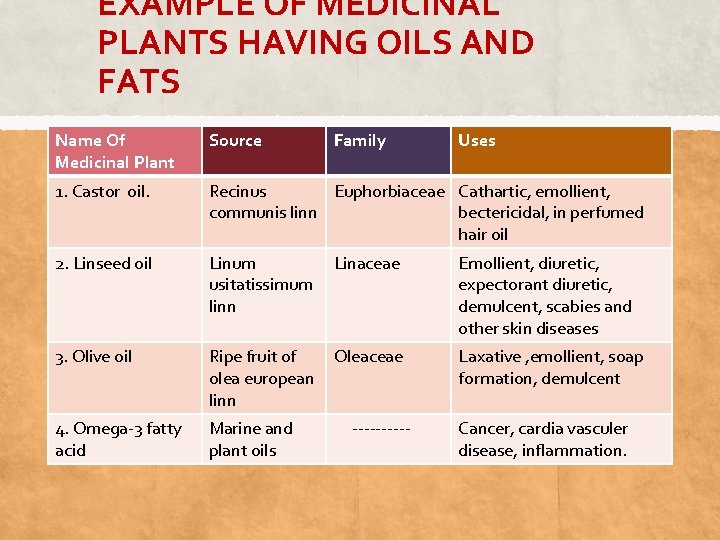
EXAMPLE OF MEDICINAL PLANTS HAVING OILS AND FATS Name Of Medicinal Plant Source Family 1. Castor oil. Recinus Euphorbiaceae Cathartic, emollient, communis linn bectericidal, in perfumed hair oil 2. Linseed oil Linum Linaceae usitatissimum linn Emollient, diuretic, expectorant diuretic, demulcent, scabies and other skin diseases 3. Olive oil Ripe fruit of Oleaceae olea european linn Laxative , emollient, soap formation, demulcent 4. Omega-3 fatty acid Marine and plant oils Cancer, cardia vasculer disease, inflammation. ----- Uses


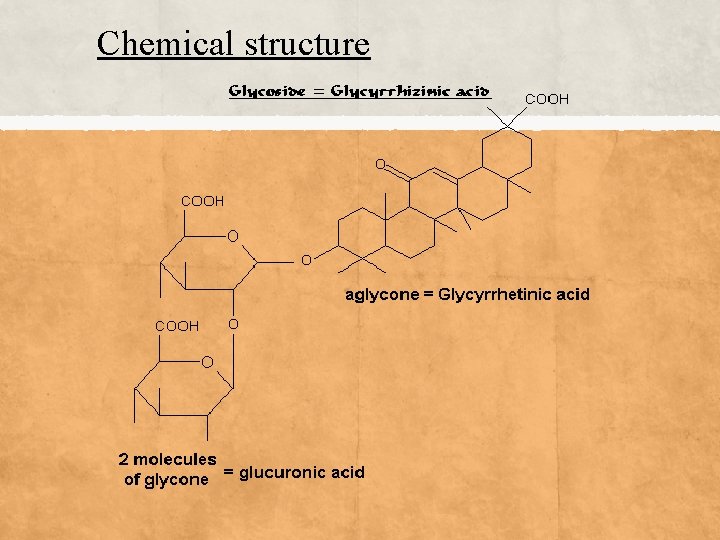
Glycosides Definition: Glycosides are a class of molecules in which, a sugar molecule (glycone) is bonded to a "non-sugar" (aglycone) molecule. Many plants store medicinally important chemicals in the form of inactive glycosides. The non-sugar portion contains the biochemically active properties of medical interest. Classification of glycosides according to a glycone part : 1 -Anthracene ------- anthraquinone glycoside 2 -Steroid ------- steroidal glycoside (cardiac) 3 -Triterpenoid –------ saponin glycoside

Chemical structure

Chemical properties 1. Separation between glycosides parts: Glycosides Hydrolysis +HCLdil Filtration glycone +aglycone +HCL Neutralization by Using alkaline (H 2 O+G)+A chloroform G + A +salt+H 2 O (H 2 O+G)+(chloroform+ A) We can separate them by using separatory funnel The best solvent to extract aglycone is Ethyl acetate because: i- immiscible in water. ii- always presents in the upper layer. 2. Glycosides also hydrolyzed by temperature or by using enzymes 3. They are water soluble compounds, insoluble in organic solvents. when a glycosides has a lot of sugars its solubility in water decrease.

Toxicology q Therapeutic use of herbal cardiac glycosides continues to be a source of toxicity today. q Recently, D lanata was mistakenly substituted for plantain in herbal products marketed to cleanse the bowel; human toxicity resulted. q Cardiac glycosides have been also found in Asian herbal products and have been a source of human toxicity.

Identification test(s) (qualitative + quantitative) A. Colorless, solid, amorphous, nonvolatile B. Give positive reaction (after hydrolysis) with 1. Molisch's solution test 2. Fehling's solution test

Extraction procedure Always glycosides founded in the plant with the enzymes which hydrolyzed them. First damage these enzymes first to extract these glycoside by the following steps: 1 -drying the plants fresh in special oven at 100 c for 30 minutes. 2 -boiling them with organic solvents for 20 minutes 3 - boiling them with acetone 5 minutes If present in this plant tannins or resins we add lead acetate to precipitate them.

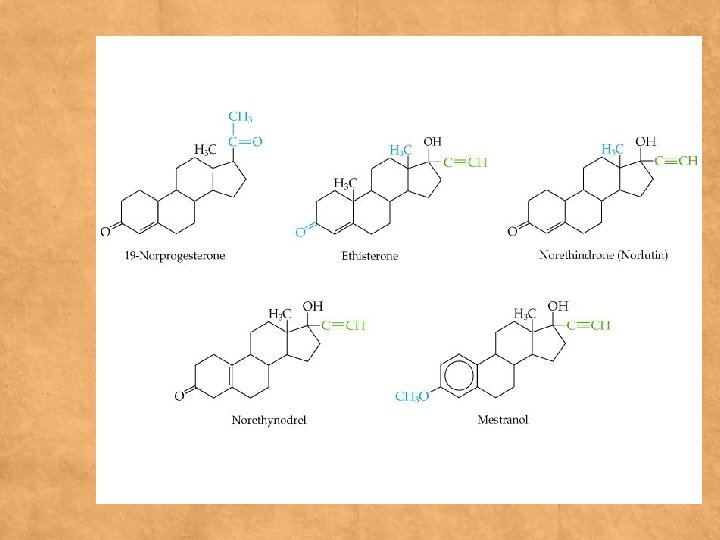
Examples (medicinal plants) 1 -Salicin –salix 2 -Cascaroside _cascara 3 -Aloin- Aloe vera 4 - Sennoside – senna 5 -Frangulin – frangula 6 - Glycyrrhizin – glycyrrhiza

Known bioactivities Ø Local irritant group: a-Sinigrin(Black mustered seeds_Brassica nigra) b-Sinalbin(White mustered seeds_Brasica alba) Ø Analgesics and antipyretics: Salicin Salisylic acid - Willow or Salix bark. Ø Keeping elasticity of blood vessels like: Rutin_Rutoside (Bitter orange peels, Lemon peels) Ø Anti-inflammatory group: a- Aloin for 1)acne 2)peptic ulcer b-Glycyrrhizin

Phyto-constituents of Medicinal Plants: Steroids Muhammad Qasim Hayat

What are Steroids? ▪ Steroids are artificial compounds, which have a similar composition to the natural male hormone testosterone. ▪ Commonly referred to as anabolic(“to build” ) or androgenic (“masculinizing” )steroids.

History of steroids ▪ Developed in the 1030’s to prevent the atrophy or break down of muscles in patients. ▪ Nazi doctors gave steroids to their soldiers in an attempt to make them more aggressive. ▪ The soviet union then decided to give steroids to their athletes. Once the U. S learned the soviet's secret, the also began giving steroids to their athletes, starting in the 1950’s

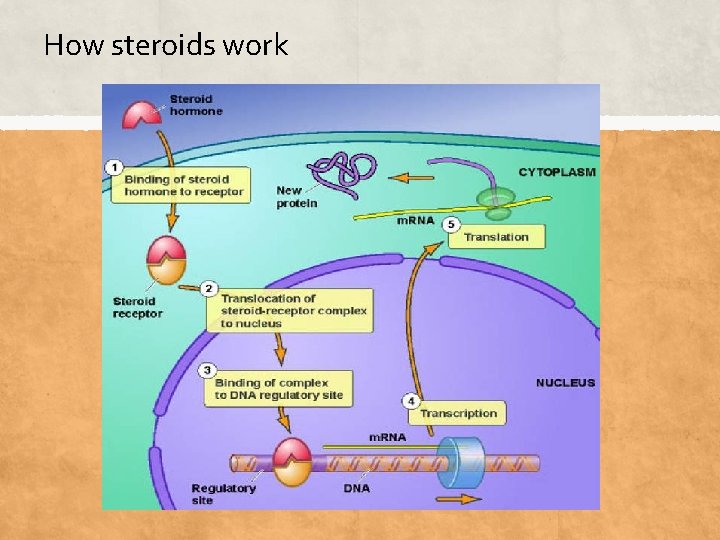
How steroids work ▪ Anabolic steroids work by stimulating the anabolic effects by binding or plugging into protein receptors in or on the cells that help create new proteins in the cells. ▪ Anabolic steroids are taken orally, by injection, or with creams and patches

How steroids work

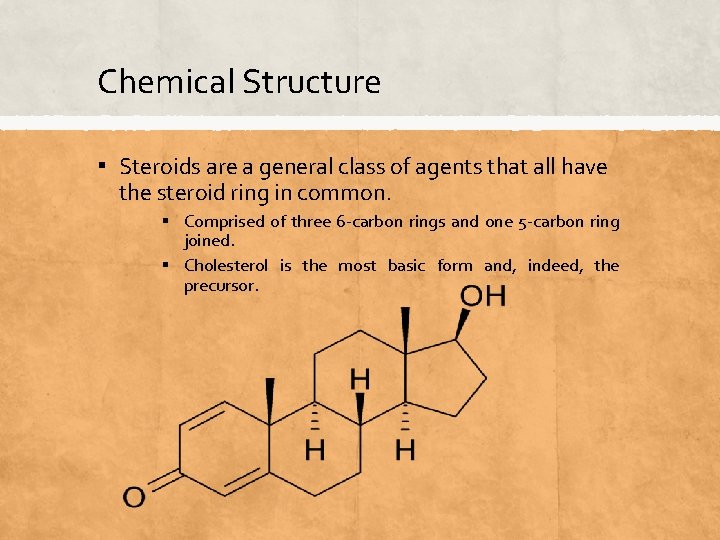
Chemical Structure ▪ Steroids are a general class of agents that all have the steroid ring in common. ▪ Comprised of three 6 -carbon rings and one 5 -carbon ring joined. ▪ Cholesterol is the most basic form and, indeed, the precursor.

▪ Shape very important for biological activity ▪ 3 D determined by ring junction AB – CD Trans AB isomer Cis AB isomer ▪ AB-CD trans junction tend to have a flat, planar structure ▪ important for hormonal activity ▪ AB-CD cis junction are bent ▪ allows them to fit on heart / smooth muscle and blood protein receptor sites ▪ poisonous steroids – some used in heart disease



Types of steroids ▪ Corticosteroids ▪ Testosterone
![Sources 1 Dioscoreaceae yam family Dioscorea genus dicots vines sweet Sources [1] Dioscoreaceae (yam family) ▪ Dioscorea genus – dicots – vines ▪ sweet](https://slidetodoc.com/presentation_image_h/a4c50c3cf89e387e94c667513caefcb6/image-51.jpg)
Sources [1] Dioscoreaceae (yam family) ▪ Dioscorea genus – dicots – vines ▪ sweet yam – food source, very low steroid content ▪ bitter yam – Mexico, South America – high content [2] Liliaceae family ▪ monocots – Far East, Phillipines ▪ very important since these provide sapogenins for manufacture of corticosteroids ▪ Smilax or Yucca [3] Amaryllidaceae ▪ Agave sisalana sisal leaf, East Africa ▪ can be used when supply of [1] and [2] short or too expensive ▪ Solanum sp. contain steroidal saponins [4] Solanaceae ▪ as well as tropane alkaloids, atropine, etc ▪ eg tomato, potato, woody nightshade [5] Scrophulariaceae ▪ Digitalis seeds full of steroids, rich source [6] Leguminosae ▪ Trigonella-foeum-graecum fengreek seed

Chemical properties ▪ Naturally occurring or synthetic fat-soluble organic compounds commonly known as lipids. ▪ Occur in animal and Plant oils and fats ▪ Have different colors like golden, yellow, blue, orange, green and even red. ▪ They are crystalline compound and contain an alcoholic group

Medicinal properties of steroid compound

Toxicology

Toxicology ▪ Examples: ▪ Corticosteriods (immunosuppressant and cause changes in the skin) ▪ Glucocorticoids (increases the risk of gastritis and peptic ulcer) ▪ Plants contain toxic Steroids ▪ Foxglove ▪ Asparagus fern ▪ Corn plant

Toxicology Foxglove Asparagus fern Corn plant

Identification tests ▪ Qualitative: ▪ Thin layer chromatography (TLC) on sulphuric acid to indicate spot position (chloroform solvent) ▪ Quantitative: ▪ i) colorimetric assay – sulphuric acid produces orange colour with steroids ▪ ii) IR spectrometry – 960 cm-1 ▪ need a lot of plant material ▪ iii) GLC micromethod – draw up assay with suitable standard and do many samples in one day ▪ quickest ▪ qualitative and quantitative

Extraction procedure ▪ Hot water extraction ▪ 5 gm of dried finely powdered plant material was taken in a beaker and 200 ml of distilled water was added. ▪ The mixture was heated on a hot plate with continuous stirring at 30º-40ºC for 20 minutes. ▪ Then the water extract was filtered through filter paper and the filtrate was used for the phytochemical analysis. ▪ The water extract was kept in refrigerator when not in use.

Extraction procedure ▪ Solvent extraction ▪ Crude plant extract was prepared by Soxhlet extraction ▪ ▪ ▪ method. About 20 gm of powdered plant material was uniformly packed into a thimble and extracted with 250 ml of different solvents separately. Solvents used were methanol, and acetone. The process of extraction continues for 24 hours or till the solvent in siphon tube of an extractor become colorless. After that the extract was taken in a beaker and kept on hot plate and heated at 30 -40ºC till all the solvent got evaporated. Dried extract was kept in refrigerator at 4ºC for their future use in phytochemical analysis.

Examples (medicinal plants)

Known activities ▪ Anabolic steroids (by structurally altering testosterone) treat certain disorders of the blood, bone mass deterioration, protein wasting states, and as a replacement therapy for male children deficient in testosterone and muscle stimulant. ▪ Corticosteroids which act as anti-inflammatory


Course: Medicinal Plants Coumarins & Tannins Muhammad Qasim Hayat

Phytochemicals ▪ Non nutritive chemical substances giving medicinal value to the plants and that produce a definite physiological action on the human body.

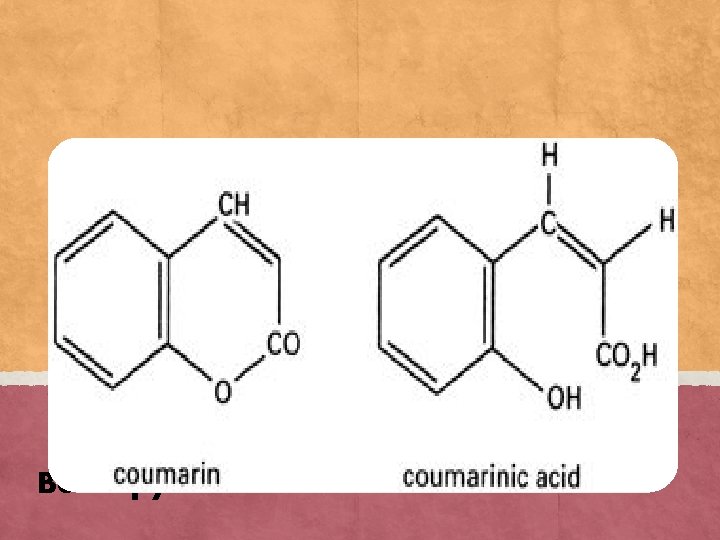
Coumarin : A natural volatile active compound found in many plants. At ambient temperature it is a white crystal with a smell similar to that of vanilla and melting point of 341 -344 K. Tannin: An astringent, bitter plant polyphenolic compound that binds to and precipitates proteins and various other organic compounds including amino acids and alkaloids.

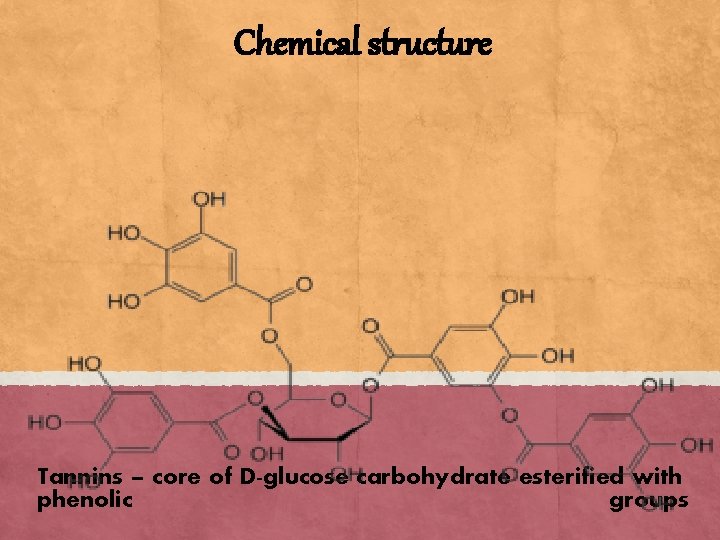
Chemical structure Tannins – core of D-glucose carbohydrate esterified with phenolic groups

Benzopyrone

Chemical Properties of Tannins ▪ Obtained as an amorphous fluffy or dense powder, yellowish-white to light-brown in color. ▪ Essentially with no odor, and a strongly astringent taste. ▪ Commercial tannic acid contains many ester linkages and is hydrolysable in the presence of acids, alkalies, or enzymes.

Chemical properties of Coumarins ▪ Coumarins are colourless or yellow crystals, soluble in organic solvents and insoluble in water. ▪ In ammoniacal solution these compounds have a blue, blue-green or violet fluorescence. ▪ While heated to 180ºC they sublime.

Extraction Procedure ▪ Maceration ▪ Percolation ▪ Ultrasound assisted extraction ▪ Microwave assisted extraction ▪ Column Chromatography

Qualitative Identification Test Tannins: 2 ml of extract was added to few drops of 1% lead acetate. A yellowish precipitate indicated the presence of tannins. Coumarins: In a test tube one g of plant sample was placed and covered with filter paper moistened with dil. Na. OH , then heated on water bath for a few minutes. The filter paper was examined under UV light, yellow fluorescence is indicative for the presence of coumarins

Quantitative Identification Test ▪ High Performance Chromatography Liquid ▪ Spectroscopic Characterization

Medicinal plants Coumarins ▪ Mellilotus officinalis (Cardioprotective) ▪ Mikania glomerata (Respiratory Diseases) Tannins ▪ Ageratum conyzoides (antibacterial)

Medicinal properties Coumarins: • Precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals. • Anti-tumor, anti-hypertension, antiarrhythmia, anti-inflammatory, antiosteoporosis, antiseptic, and analgesic.

Tannins ▪ Important ingredient in the process of tanning leather. ▪ When incubated with red grape juice and red wines with a high content of condensed tannins, the poliovirus, herpes simplex virus, and various enteric viruses are inactivated.

▪ Tannins have potential antiviral, antibacterial antiparasitic effects. shown and ▪ Effective in protecting the kidneys

Toxicology ▪ Coumarin is moderately toxic to the liver and kidneys, with a "Median Lethal Dose" (LD 50) of 275 mg/kg. ▪ A potent rodenticide that can cause internal hemorrhage and death. ▪ USFDA banned coumarin direct use as human food, as reported that coumarin content of alcoholic beverages cause headaches.

▪ Coumarin is subject to restrictions on its use in perfumery as some people may become sensitised. ▪ A tolerable daily intake established by the German Federal Assessment is body weight. 0. 1 Institute for Risk mg coumarin per kg

▪ Tannins negatively affect an animal's feed intake, feed digestibility, and efficiency of production ▪ These effects vary depending on the content and type of tannin ingested and on the animal's tolerance ▪ Tannin toxicity to rumen microorganisms has been described for several bacteria species such as Streptococcus bovis, Butyvibrio fibrosolvens, Fibrobacter succinogenes, Prevotella ruminicola, and Ruminobacter amylophilis.

Monogastrics animals fed diets with a level of tannins under experience 5% ▪ depressed growth rates, ▪ low protein utilization, ▪ damage to the mucosal lining of the digestive tract, ▪ alteration in the excretion of certain cations, and ▪ increased excretion of proteins and essential amino acids. In poultry, small quantities of tannins in the diet cause adverse effects ▪ levels from production, ▪ levels from 0. 5 to 2. 0% can cause depression in growth and egg 3 to 7% can cause death.

▪ Thank You
 Hayat ve hayat dışı sigortalar arasındaki farklar
Hayat ve hayat dışı sigortalar arasındaki farklar Ur
Ur Qasim amin
Qasim amin Qasim and yahya limited
Qasim and yahya limited Objective of medicinal plants
Objective of medicinal plants Importance of medicinal plants
Importance of medicinal plants Wa ta izzu man tasha in arabic text
Wa ta izzu man tasha in arabic text Sami allahu liman hamidah meaning
Sami allahu liman hamidah meaning Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Alkaloids definition pharmacognosy
Alkaloids definition pharmacognosy Pharmacognosy alkaloids
Pharmacognosy alkaloids Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Ergoline alkaloids
Ergoline alkaloids Alkaloid examples
Alkaloid examples Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Ephedra
Ephedra Protoalkaloid
Protoalkaloid Purine alkaloids
Purine alkaloids Peomus
Peomus Purine examples
Purine examples Belladonna alkaloids drugs
Belladonna alkaloids drugs Tropane alkaloids
Tropane alkaloids Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Tocolytic drugs
Tocolytic drugs Nonvascular plants
Nonvascular plants Non vascular vs vascular plants
Non vascular vs vascular plants Flowering plants and non flowering plants similarities
Flowering plants and non flowering plants similarities C3 plants vs c4 plants
C3 plants vs c4 plants Jewel weed medicinal
Jewel weed medicinal Mixtures of organic substances and a medicinal agent are:
Mixtures of organic substances and a medicinal agent are: Advanced medicinal chemistry
Advanced medicinal chemistry Tsaang gubat preparation
Tsaang gubat preparation Quality control methods for medicinal plant materials
Quality control methods for medicinal plant materials Patrick an
Patrick an Drug receptor interaction medicinal chemistry
Drug receptor interaction medicinal chemistry Toilet preparation act 1955
Toilet preparation act 1955 Gas medicinal
Gas medicinal Veterinary medicinal product dossier
Veterinary medicinal product dossier Farmacocinetica
Farmacocinetica Drug receptor interaction medicinal chemistry
Drug receptor interaction medicinal chemistry Medicinal algae
Medicinal algae Vanilla processing
Vanilla processing Farmacologia
Farmacologia Peter wipf pitt
Peter wipf pitt Medicinal algae
Medicinal algae Quinoline electrophilic substitution
Quinoline electrophilic substitution Pyrrole medicinal uses
Pyrrole medicinal uses Cosechadora de stevia
Cosechadora de stevia Burmm
Burmm Bachelorkontrakt
Bachelorkontrakt Vejetasyon tipleri
Vejetasyon tipleri Insurans hayat tingkatan 5
Insurans hayat tingkatan 5 Kitaran hayat rekod
Kitaran hayat rekod Adli olgu nedir
Adli olgu nedir Tasarım çeşitleri 7. sınıf
Tasarım çeşitleri 7. sınıf Jumlah fasa yang terlibat dalam pembangunan atur cara
Jumlah fasa yang terlibat dalam pembangunan atur cara Osmanlıcılık nedir
Osmanlıcılık nedir Karamand
Karamand Mamul karması
Mamul karması Kitaran hayat projek
Kitaran hayat projek Hayat bilgisi dersi nedir
Hayat bilgisi dersi nedir 8. sınıf dinin temel gayesi slayt
8. sınıf dinin temel gayesi slayt Hayat sahası politikası
Hayat sahası politikası 1968 hayat bilgisi programı
1968 hayat bilgisi programı Hayat holding kastamonu entegre
Hayat holding kastamonu entegre Sürünücü gövde ile üreme
Sürünücü gövde ile üreme Kitaran hayat pembangunan sistem
Kitaran hayat pembangunan sistem Kaedah sifat
Kaedah sifat Dr ashik hayat
Dr ashik hayat Yazm
Yazm Bahrul hayat
Bahrul hayat Mamulün hayat seyri grafiği
Mamulün hayat seyri grafiği Etk ne demek
Etk ne demek Siber zorbalik
Siber zorbalik Zararlı programlara karşı alınacak tedbirler
Zararlı programlara karşı alınacak tedbirler Su varsa hayat var
Su varsa hayat var Ilkyardımın a b c si nedir
Ilkyardımın a b c si nedir Hamza hayat
Hamza hayat Bep toplantı tutanağı örneği
Bep toplantı tutanağı örneği Half brick wall in stretcher bond report
Half brick wall in stretcher bond report Course title and course number
Course title and course number Course interne moyenne externe
Course interne moyenne externe