Counting Atoms Review Science 10 The symbol of

Counting Atoms Review Science 10
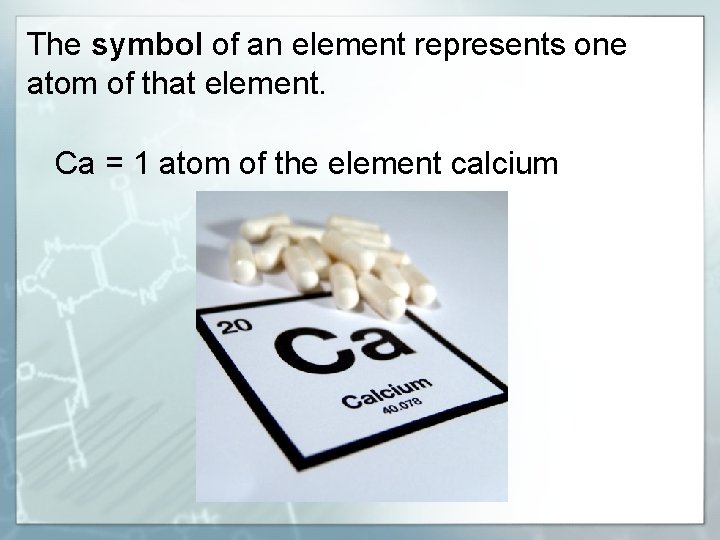
The symbol of an element represents one atom of that element. Ca = 1 atom of the element calcium
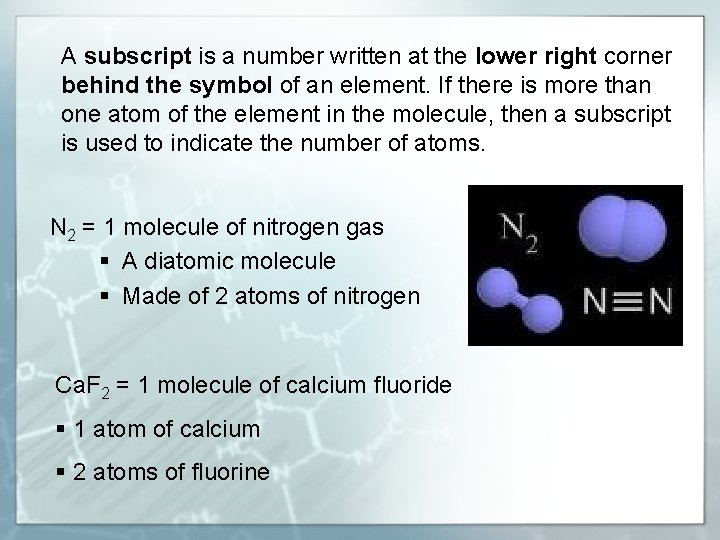
A subscript is a number written at the lower right corner behind the symbol of an element. If there is more than one atom of the element in the molecule, then a subscript is used to indicate the number of atoms. N 2 = 1 molecule of nitrogen gas § A diatomic molecule § Made of 2 atoms of nitrogen Ca. F 2 = 1 molecule of calcium fluoride § 1 atom of calcium § 2 atoms of fluorine

A subscript outside the bracket multiplies all the elements inside the brackets. Ba 3(PO 4)2 = 1 molecule of barium phosphate § 3 atoms of barium § 2 atoms of phosphorus § 8 atoms of oxygen § Total number of atoms = 13

A coefficient is a number written in front of a chemical symbol and indicates the number of atoms of that element. 3 C = 3 atoms of the element carbon
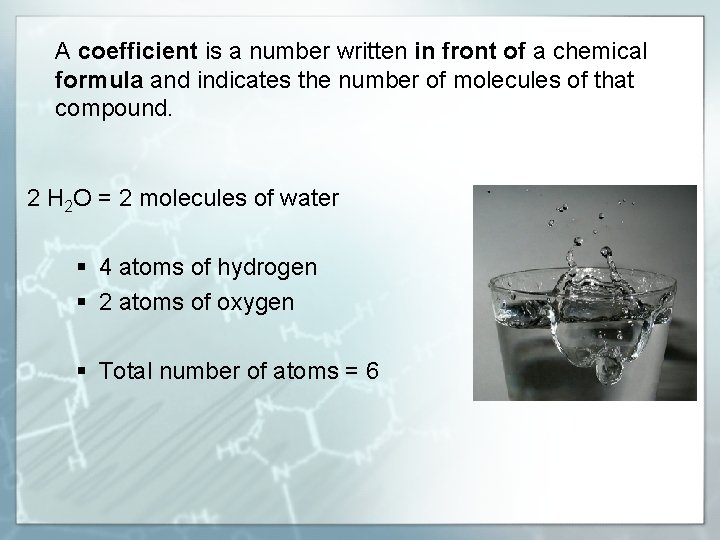
A coefficient is a number written in front of a chemical formula and indicates the number of molecules of that compound. 2 H 2 O = 2 molecules of water § 4 atoms of hydrogen § 2 atoms of oxygen § Total number of atoms = 6

3 Fe. SO 4 = 3 molecules of iron (II) sulfate § 3 atoms of iron § 3 atoms of sulfur § 12 atoms of oxygen § Total number of atoms = 18

4 Cu(NO 3)2 q 4 molecules of copper (II) nitrate § 4 atoms of copper § 8 atoms of nitrogen § 24 atoms of oxygen § Total number of atoms = 36

Credits q q q http: //commons. wikimedia. org/wiki/File: Natrium. jpg http: //upload. wikimedia. org/wikipedia/commons/6/6 e/Fosfore%C 4%8 Dnan_ho%C 5 %99 e%C 4%8 Dnat%C 3%BD. PNG http: //commons. wikimedia. org/wiki/File: C, 6. jpg http: //upload. wikimedia. org/wikipedia/commons/f/f 3/Uhl%C 3%ADk. PNG http: //commons. wikimedia. org/wiki/File: Lead(II)_nitrate_1. jpg http: //commons. wikimedia. org/wiki/File: Cu. SO 4. JPG
- Slides: 9