Counting Atoms How many atoms are in Na












- Slides: 34

Counting Atoms • • • How many atoms are in: Na. Cl? H 2 O? Mg. SO 4? Pb(SO 4)2? • We can’t actually count atoms in chemistry! b/c they are too small!! • So what can we count? ?

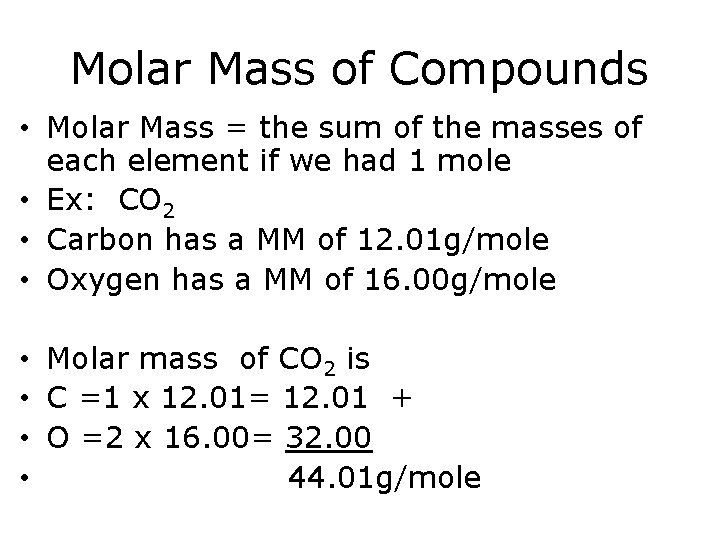
Molar Mass of Compounds • Molar Mass = the sum of the masses of each element if we had 1 mole • Ex: CO 2 • Carbon has a MM of 12. 01 g/mole • Oxygen has a MM of 16. 00 g/mole • Molar mass of CO 2 is • C =1 x 12. 01= 12. 01 + • O =2 x 16. 00= 32. 00 • 44. 01 g/mole

Molar Mass • Find the Molar Masses of the following compounds. 1. Water 2. Sodium chloride (Salt) 3. Sodium bicarbonate (Baking Soda) 4. Glucose

1. Water 2. Sodium chloride (Salt)

3. Sodium bicarbonate (Baking Soda) 4. Glucose

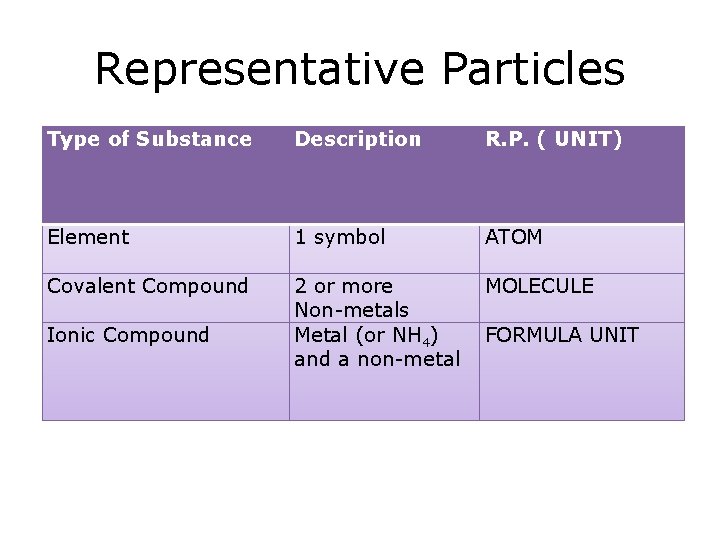
Representative Particles Type of Substance Description R. P. ( UNIT) Element 1 symbol ATOM Covalent Compound 2 or more Non-metals Metal (or NH 4) and a non-metal MOLECULE Ionic Compound FORMULA UNIT

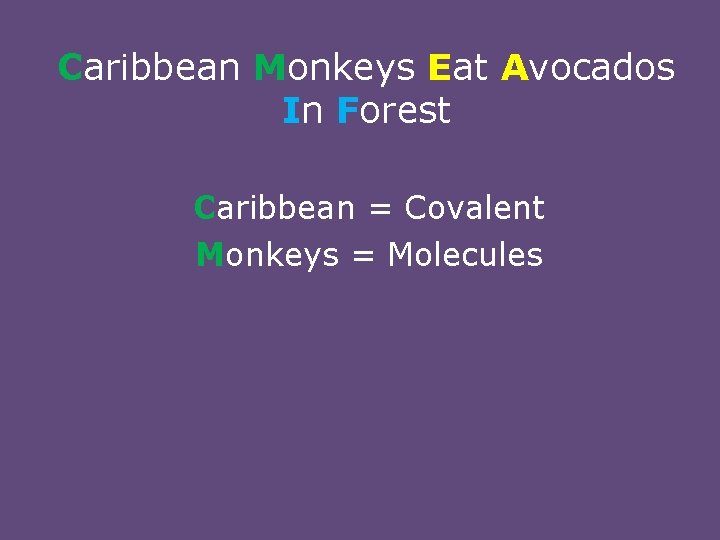
Caribbean Monkeys Eat Avocados In Forest Caribbean = Covalent Monkeys = Molecules

Caribbean Monkeys Eat Avocados In Forest Eat = Element Avocados = Atoms

Caribbean Monkeys Eat Avocados In Forest In = Ionic Forest = Formula Unit

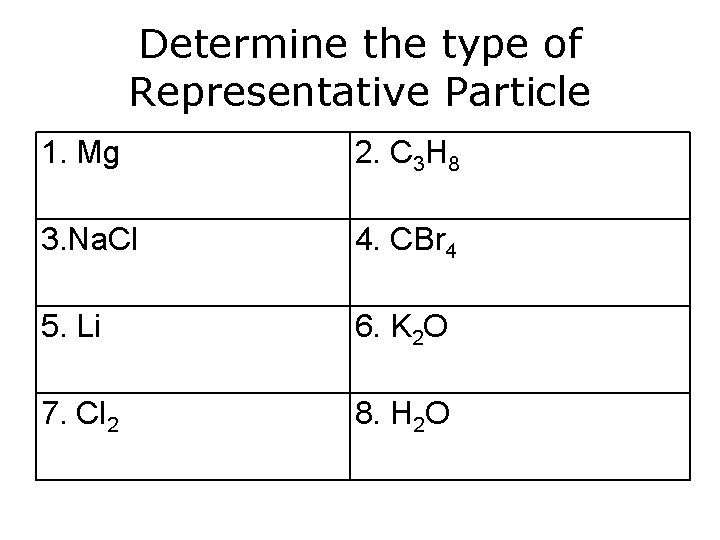
Determine the type of Representative Particle 1. Mg 2. C 3 H 8 3. Na. Cl 4. CBr 4 5. Li 6. K 2 O 7. Cl 2 8. H 2 O

Welcome to St. Mole Islands!!! http: //www. youtube. com/watch? v=i 5 Cg 3 J 8 q 9 i 8


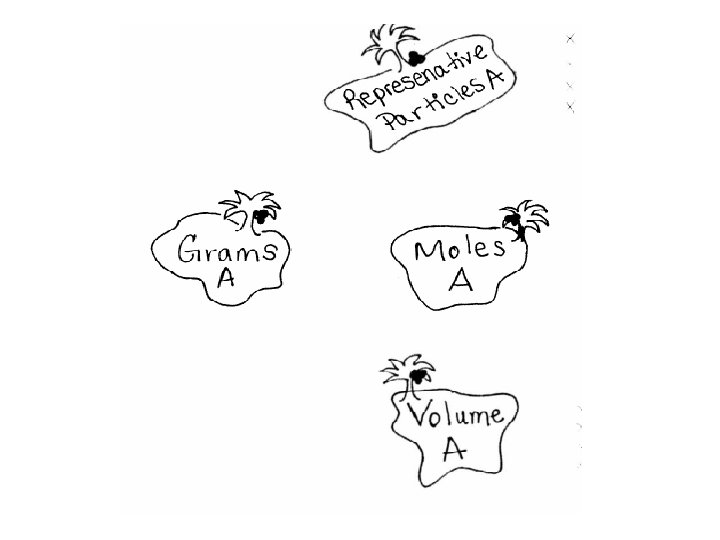
To go anywhere on the mole islands you must go thru the mole!!! 1 mole = 6. 02 x 1023 atom or 1 mole = 6. 02 x 1023 molecule or 1 mole = 6. 02 x 1023 formula unit



Example Problems Using Representative Particles

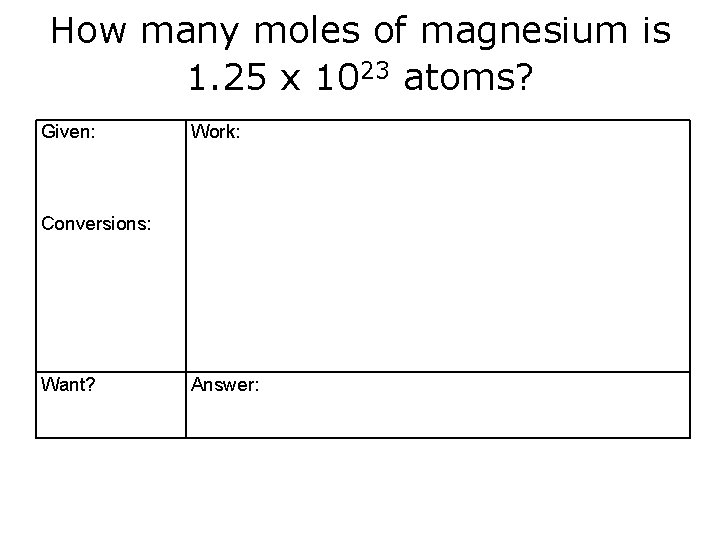
How many moles of magnesium is 1. 25 x 1023 atoms? Given: Work: Conversions: Want? Answer:

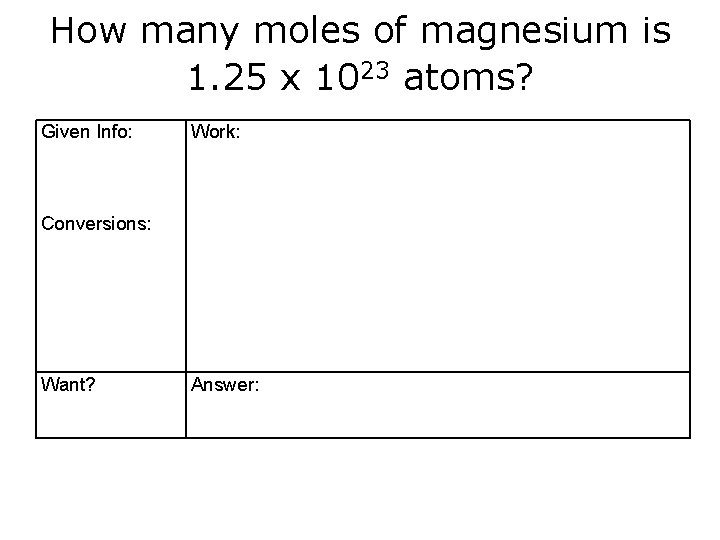
How many moles of magnesium is 1. 25 x 1023 atoms? Given Info: Work: Conversions: Want? Answer:

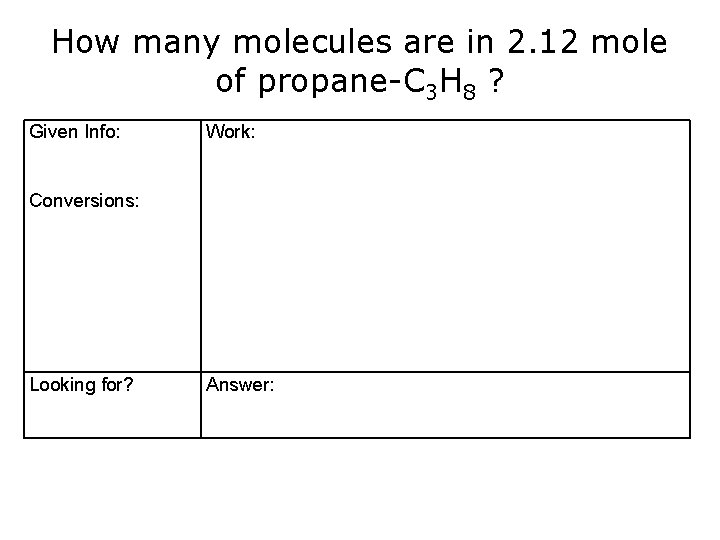
How many molecules are in 2. 12 mole of propane-C 3 H 8 ? Given Info: Work: Conversions: Looking for? Answer:

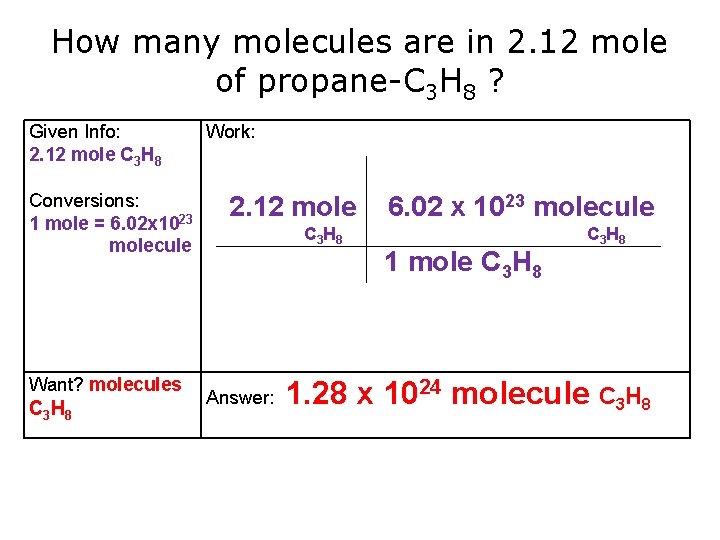
How many molecules are in 2. 12 mole of propane-C 3 H 8 ? Given Info: 2. 12 mole C 3 H 8 Conversions: 1 mole = 6. 02 x 1023 molecule Want? molecules C 3 H 8 Work: 2. 12 mole C 3 H 8 Answer: 6. 02 x 1023 molecule 1 mole C 3 H 8 C 3 H 8 1. 28 x 1024 molecule C 3 H 8

Example Problems Using Molar Mass


How many grams are in 2 moles of calcium hydroxide? Given Info: Work: Conversions: Want? Answer:

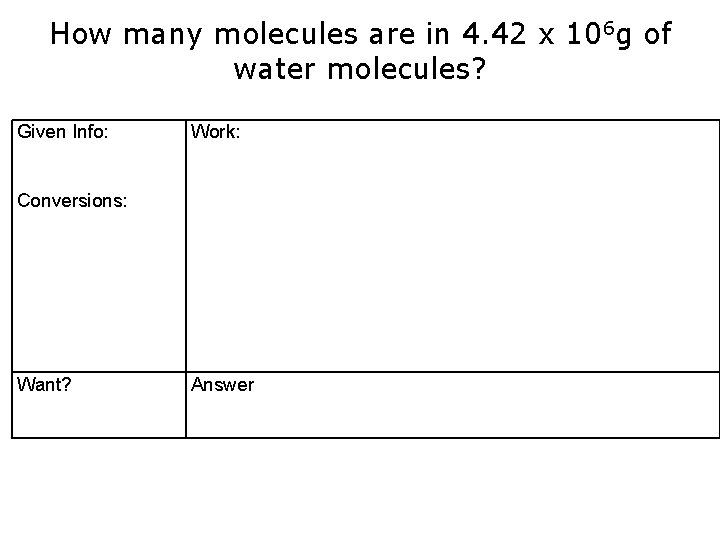
How many molecules are in 4. 42 x 106 g of water molecules? Given Info: Work: Conversions: Want? Answer


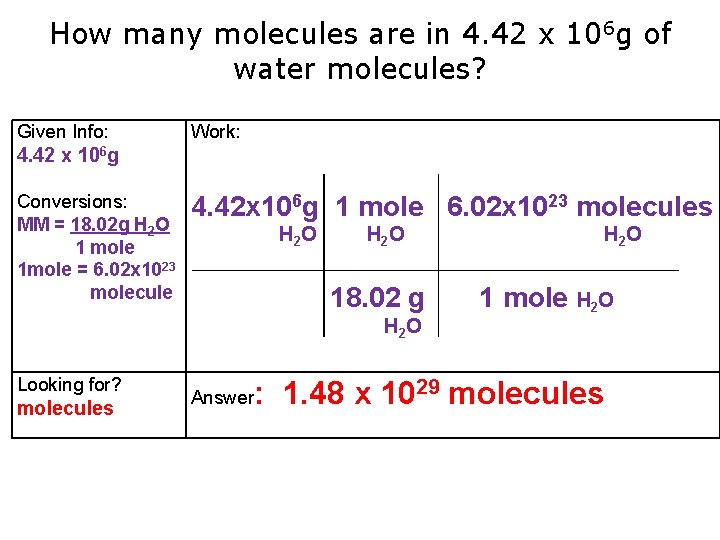
How many molecules are in 4. 42 x 106 g of water molecules? Given Info: Work: 4. 42 x 106 g Conversions: MM = 18. 02 g H 2 O 1 mole 1 mole = 6. 02 x 1023 molecule 4. 42 x 106 g 1 mole 6. 02 x 1023 molecules H 2 O 18. 02 g H 2 O Looking for? molecules Answer H 2 O 1 mole H 2 O : 1. 48 x 1029 molecules

Example Problems Using Molar Volume

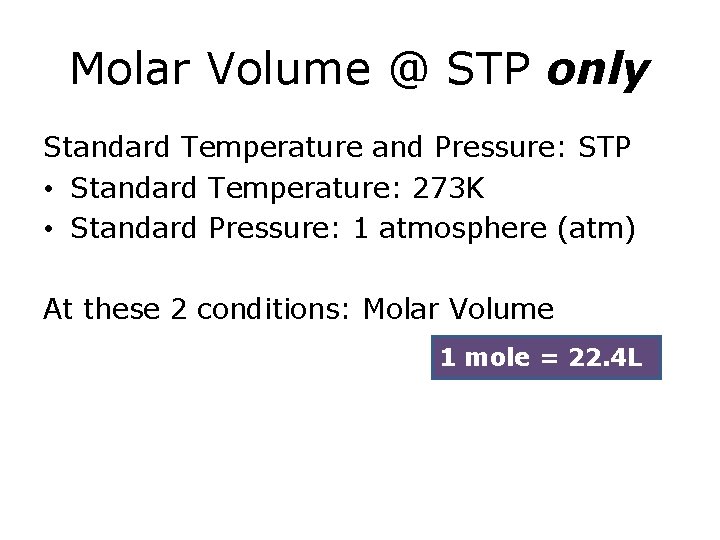
Molar Volume @ STP only Standard Temperature and Pressure: STP • Standard Temperature: 273 K • Standard Pressure: 1 atmosphere (atm) At these 2 conditions: Molar Volume 1 mole = 22. 4 L


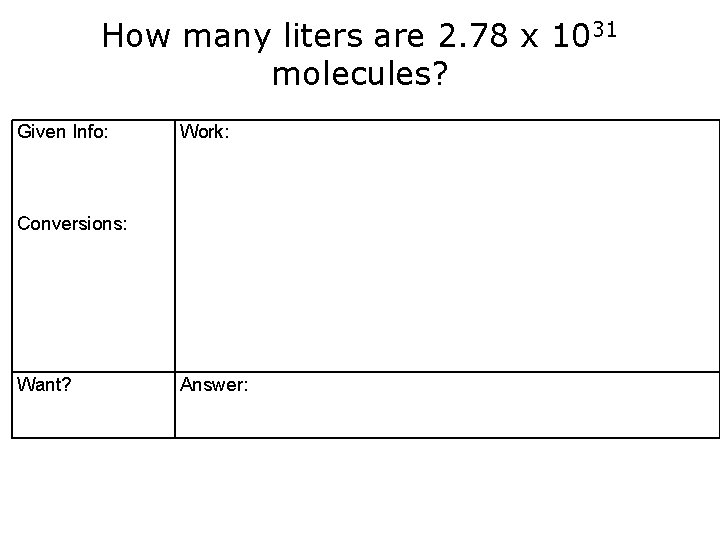
How many liters are 2. 78 x 1031 molecules? Given Info: Work: Conversions: Want? Answer:

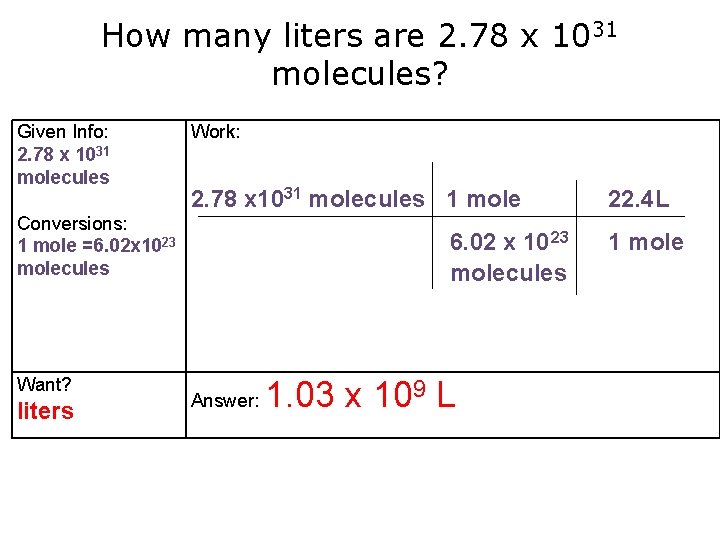
How many liters are 2. 78 x 1031 molecules? Given Info: 2. 78 x 1031 molecules Work: 2. 78 x 1031 molecules 1 mole Conversions: 1 mole =6. 02 x 1023 molecules Want? liters 6. 02 x 10 23 molecules Answer: 1. 03 x 109 L 22. 4 L 1 mole

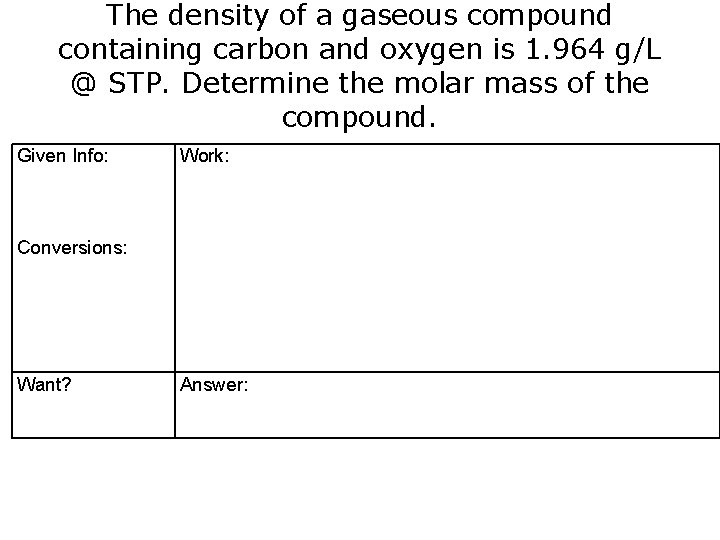
The density of a gaseous compound containing carbon and oxygen is 1. 964 g/L @ STP. Determine the molar mass of the compound. Given Info: Work: Conversions: Want? Answer:

The density of a gaseous compound containing carbon and oxygen is 1. 964 g/L @ STP. Determine the molar mass of the compound. Given Info: Work: Conversions: Want? Answer:
