Counting Atoms Counting Atoms Subscript the small after

Counting Atoms
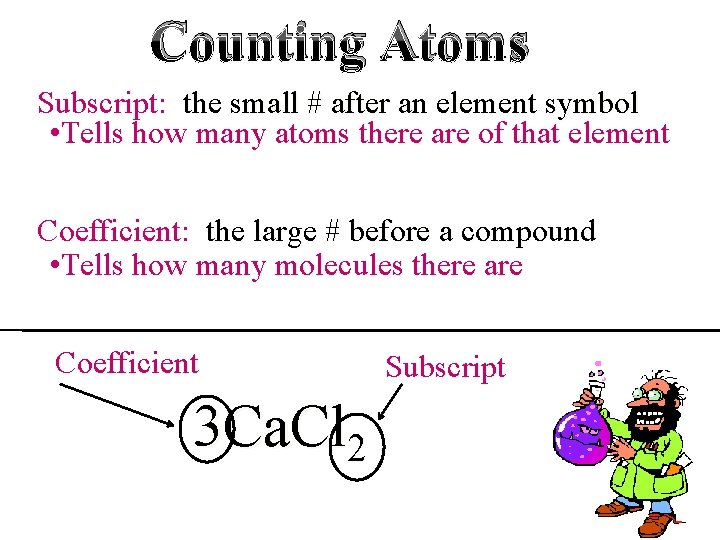
Counting Atoms Subscript: the small # after an element symbol • Tells how many atoms there are of that element Coefficient: the large # before a compound • Tells how many molecules there are Coefficient 3 Ca. Cl 2 Subscript
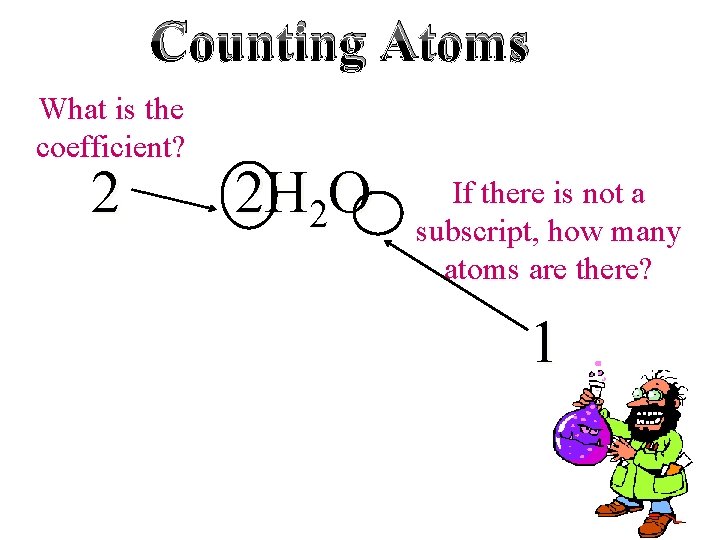
Counting Atoms What is the coefficient? 2 2 H 2 O If there is not a subscript, how many atoms are there? 1
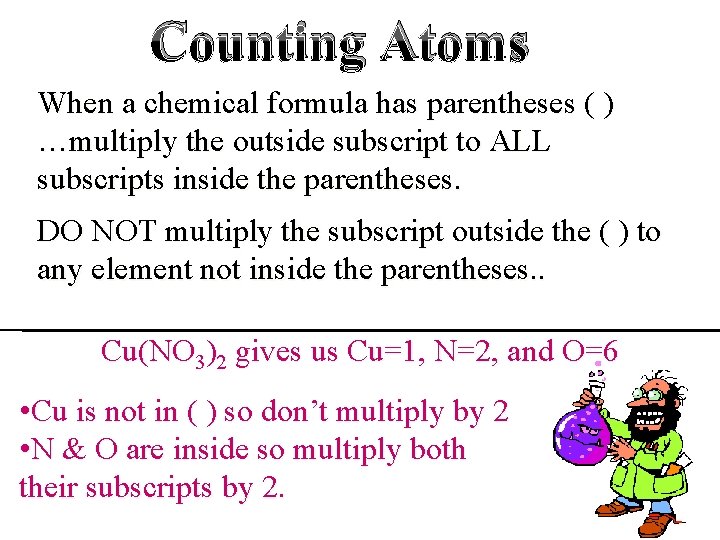
Counting Atoms When a chemical formula has parentheses ( ) …multiply the outside subscript to ALL subscripts inside the parentheses. DO NOT multiply the subscript outside the ( ) to any element not inside the parentheses. . Cu(NO 3)2 gives us Cu=1, N=2, and O=6 • Cu is not in ( ) so don’t multiply by 2 • N & O are inside so multiply both their subscripts by 2.

Counting Atoms After you figure out how many atoms in one molecule, if there is a coefficient, multiply by that #. 3 Ca. Cl 2 • In one molecule, there is Ca=1 and Cl=2, multiply this by the coefficient to get TOTAL in all 3 molecules • 3 x 3 = 9 totals atoms

Counting Atoms Try this one!!! 4 Cu(NO 3)2 • How many Cu in one molecule? 1 • How many N in one molecule? 2 • How many O in one molecule? 6 • How many total atoms in this molecule? 9 x 4 = 36
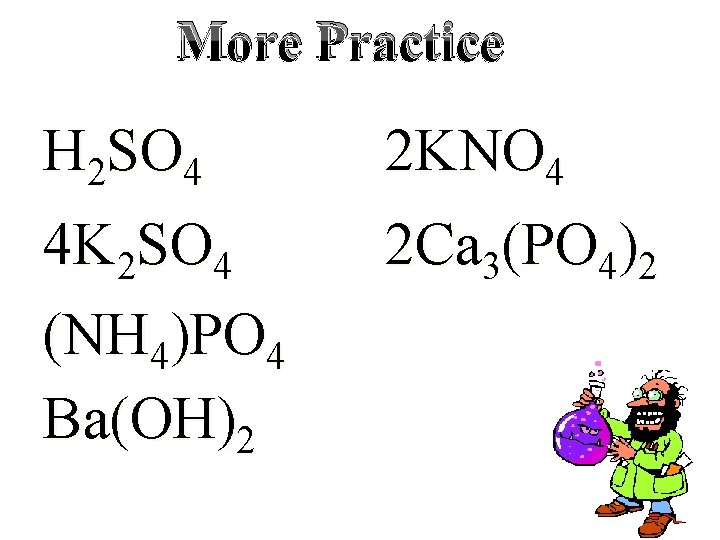
More Practice H 2 SO 4 2 KNO 4 4 K 2 SO 4 (NH 4)PO 4 Ba(OH)2 2 Ca 3(PO 4)2
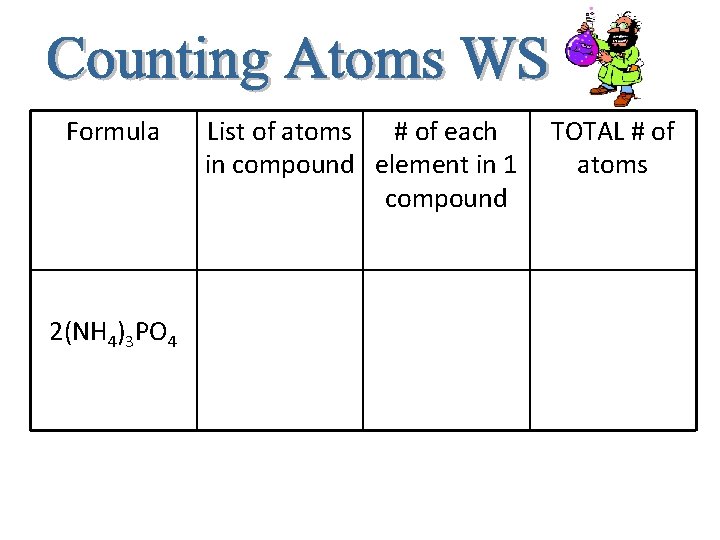
Formula 2(NH 4)3 PO 4 List of atoms # of each in compound element in 1 compound TOTAL # of atoms
- Slides: 8