Counting Atoms and Balancing Equations TRUE or FALSE

Counting Atoms and Balancing Equations
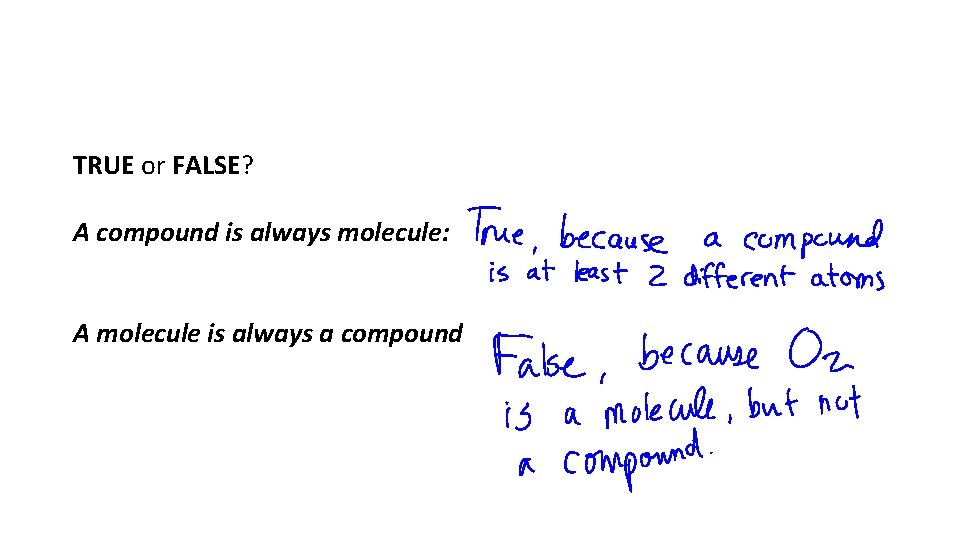
TRUE or FALSE? A compound is always molecule: A molecule is always a compound
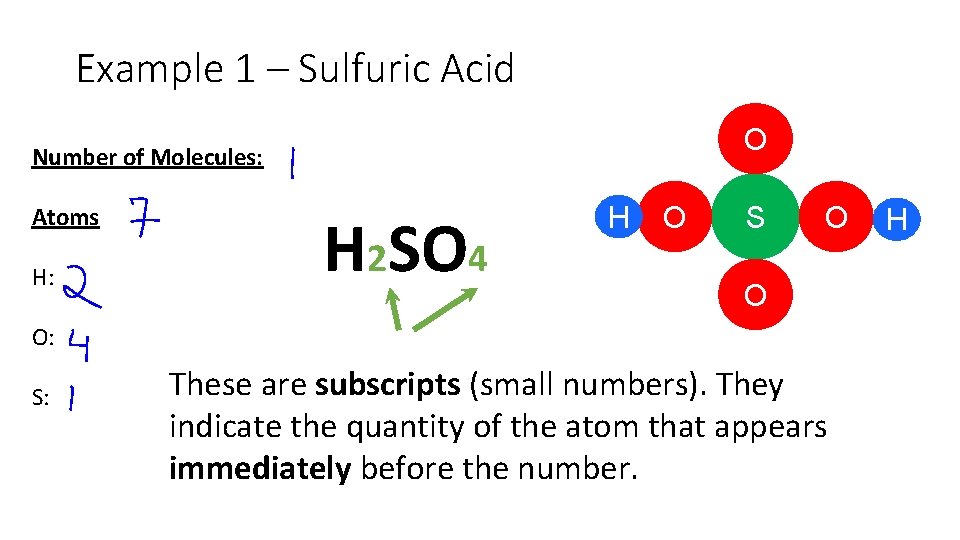
Example 1 – Sulfuric Acid O Number of Molecules: Atoms H: H 2 SO 4 H O S O O O: S: These are subscripts (small numbers). They indicate the quantity of the atom that appears immediately before the number. H

Example 2 – Calcium hydroxide Number of Molecules: Atoms Ca: Ca(OH)2 H O Ca O: H: Sometimes a subscript will appear after brackets. This means that all the atoms inside the brackets are multiplied by this number. O H
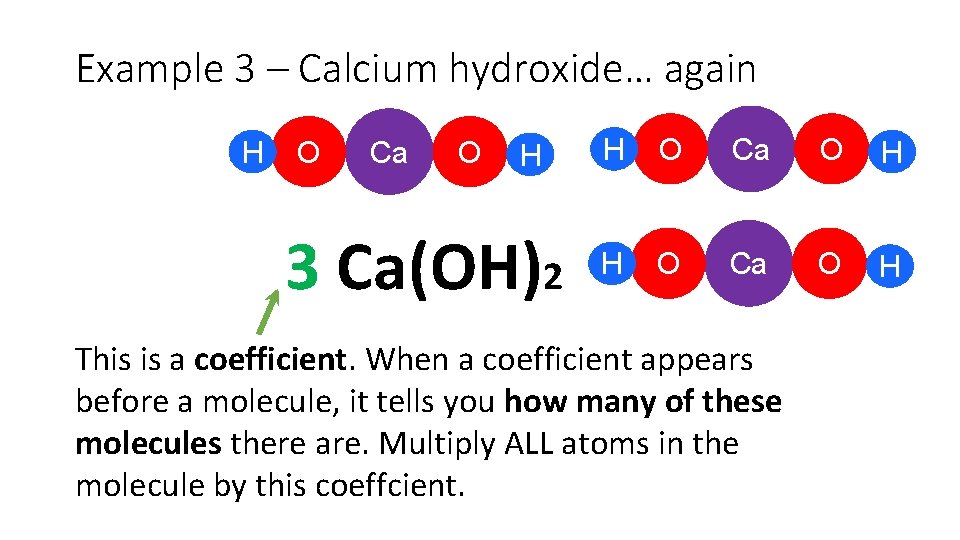
Example 3 – Calcium hydroxide… again H O Ca O H 3 Ca(OH)2 H O Ca O H This is a coefficient. When a coefficient appears before a molecule, it tells you how many of these molecules there are. Multiply ALL atoms in the molecule by this coeffcient.
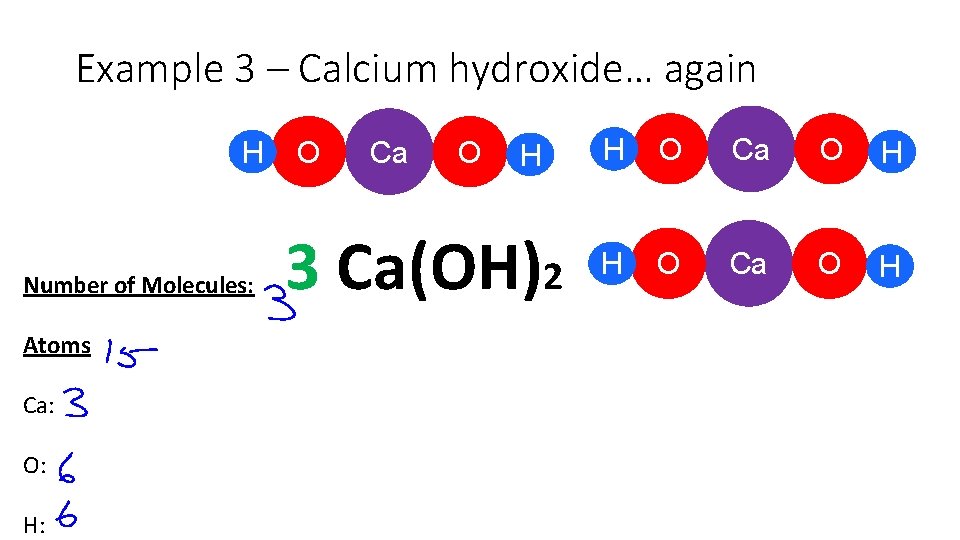
Example 3 – Calcium hydroxide… again H Number of Molecules: Atoms Ca: O: H: O Ca O H 3 Ca(OH)2 H O Ca O H
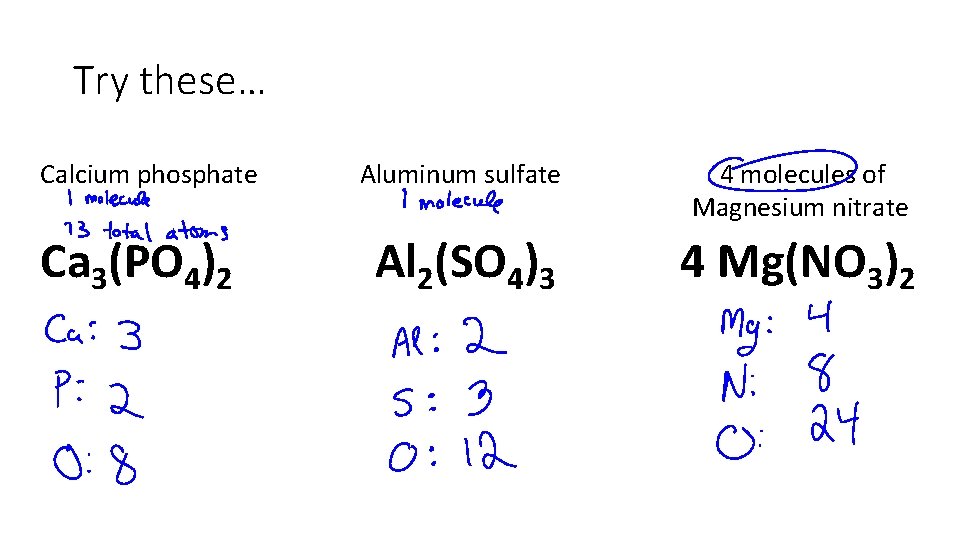
Try these… Calcium phosphate Ca 3(PO 4)2 Aluminum sulfate 4 molecules of Magnesium nitrate Al 2(SO 4)3 4 Mg(NO 3)2

Balancing Equations In a chemical reaction, atoms are rearranged. The number of atoms in the reactants MUST EQUAL the number of atoms in the products. This is the Law of Conservation of Matter: Matter is neither created nor destroyed. It is merely rearranged.
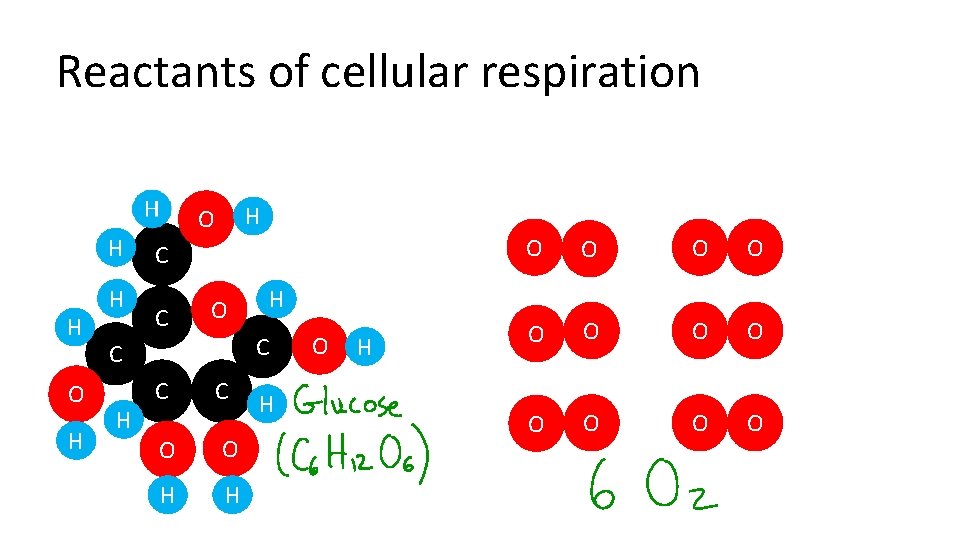
Reactants of cellular respiration H H O H H H O C C H O O H H O O O H C C O H
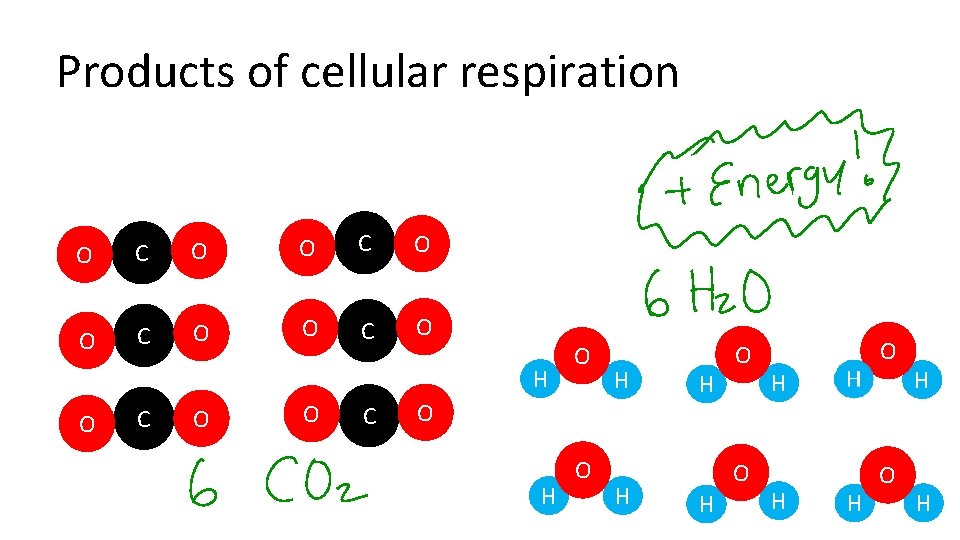
Products of cellular respiration O C O H O C O H O H H O O H H H O H H
Complete p. 14 -15
- Slides: 11