Counterfeit Drugs A CLEAR AND PRESENT DANGER State


![What is a Counterfeit Drug? According to WHO: “[A] product that is deliberately and What is a Counterfeit Drug? According to WHO: “[A] product that is deliberately and](https://slidetodoc.com/presentation_image/237d4642d79121f325181e3450965edf/image-5.jpg)

![Example 1: Lipitor According to WHO: “ [A] product that is deliberately and fraudulently Example 1: Lipitor According to WHO: “ [A] product that is deliberately and fraudulently](https://slidetodoc.com/presentation_image/237d4642d79121f325181e3450965edf/image-8.jpg)






- Slides: 15

Counterfeit Drugs: A CLEAR AND PRESENT DANGER State Senator Marvin Riegsecker, R. Ph.

Prevalence of Drug Counterfeiting Counterfeit drugs are more prevalent in developing countries. According to the World Health Organization (WHO): A. Industrialized Countries: 22% B. Developing Countries: 78% Africa: 50 -60% China: 50% Mexico, Argentina, Columbia: 40% Brazil: 30 -40% India: 15 -20%

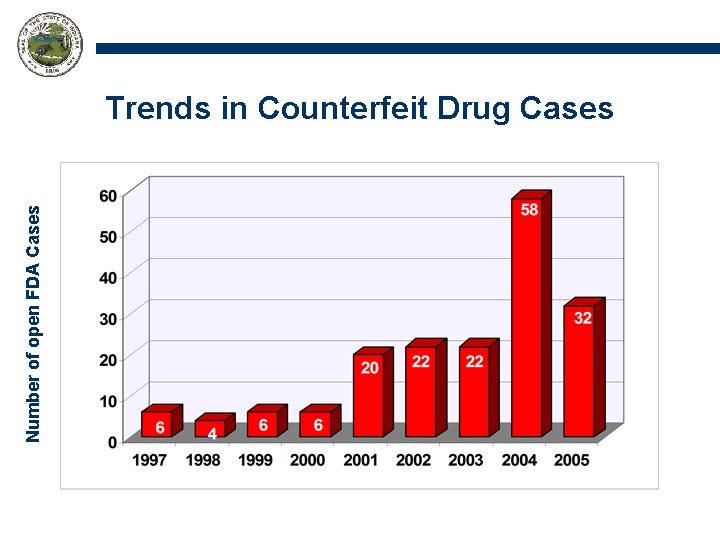
Number of open FDA Cases Trends in Counterfeit Drug Cases

![What is a Counterfeit Drug According to WHO A product that is deliberately and What is a Counterfeit Drug? According to WHO: “[A] product that is deliberately and](https://slidetodoc.com/presentation_image/237d4642d79121f325181e3450965edf/image-5.jpg)
What is a Counterfeit Drug? According to WHO: “[A] product that is deliberately and fraudulently mislabeled with respect to identity and source. ”

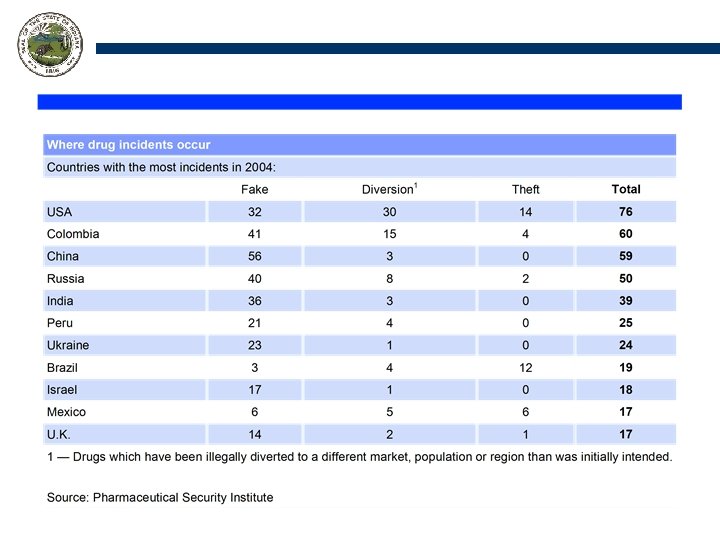
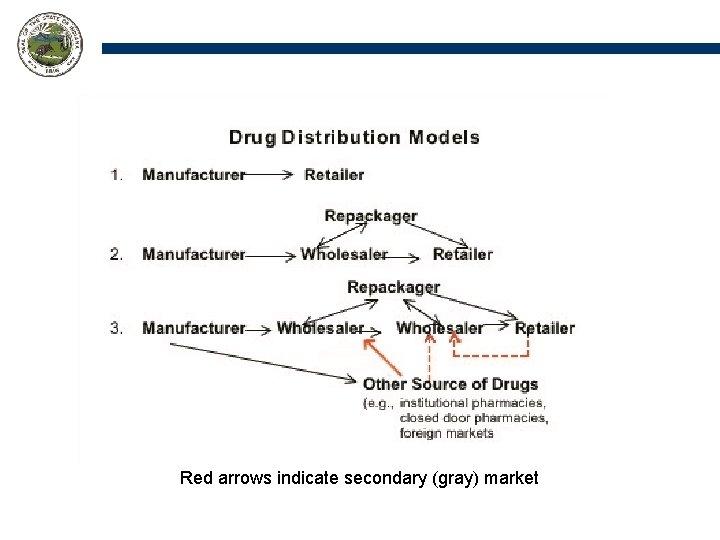
Where is the problem? Drug counterfeiting isn’t a manufacturing problem, it is a distribution problem.

Red arrows indicate secondary (gray) market
![Example 1 Lipitor According to WHO A product that is deliberately and fraudulently Example 1: Lipitor According to WHO: “ [A] product that is deliberately and fraudulently](https://slidetodoc.com/presentation_image/237d4642d79121f325181e3450965edf/image-8.jpg)
Example 1: Lipitor According to WHO: “ [A] product that is deliberately and fraudulently mislabeled with respect to identity and source. ”

Lipitor: Which pill is authentic?

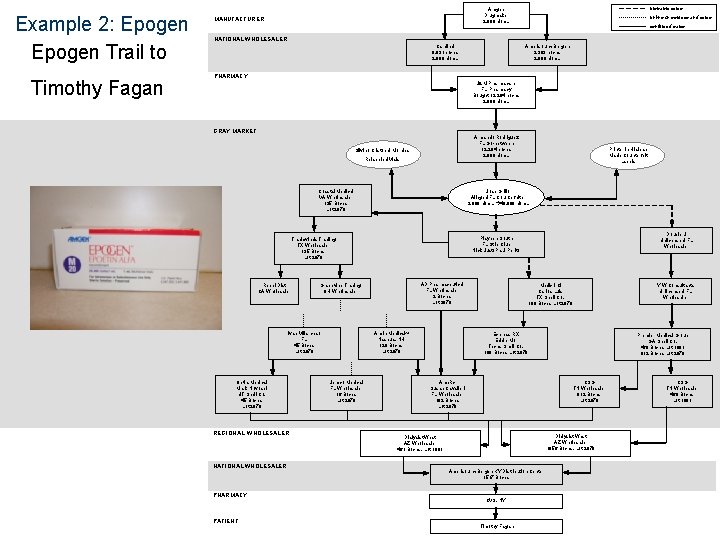
Example 2: Epogen Trail to Timothy Fagan probable sales Amgen Drugmaker 2, 000 U/m. L MANUFACTURER unknown sources and sales confirmed sales NATIONAL WHOLESALER Cardinal 8, 931 boxes 2, 000 U/m. L Amerisource. Bergen 3, 363 boxes 2, 000 U/m. L PHARMACY J&M Pharmacare FL Pharmacy Bought 12, 294 boxes 2, 000 U/m. L GRAY MARKET Armando Rodriguez FL Go-between 12, 294 boxes 2, 000 U/m. L Silvino Cristobal Morales Relabeled Vials Coastal Medical VA Wholesaler 135 Boxes Lot 2970 Jose Grillo Alleged FL Counterfeiter 2, 000 U/m. L 40, 000 U/m. L Optia Medical Mark Novosel UT Shell Co. 45 Boxes Lot 2970 REGIONAL WHOLESALER NATIONAL WHOLESALER PHARMACY PATIENT AD Pharmaceutical FL Wholesaler 2 Boxes Lot 2970 Grapevine Trading OH Wholesaler Ivan Villarchao FL 45 Boxes Lot 2970 Armin Medical 24 Nashua, NH 129 Boxes Lot 2970 Jemco Medical FL Wholesaler 16 Boxes Lot 2970 Double J Unlicensed FL Wholesaler Playpen South FL Strip Club Nick Just/ Paul Perito Tradewinds Trading TX Wholesaler 135 Boxes Lot 2970 Rebel Dist CA Wholesaler Printer in Hialeah Made Counterfeit Labels Y W Consultants Unlicensed FL Wholesaler Medix Intl Carlos Luis TX Shell Co. 180 Boxes Lot 2970 Express RX Eddie Mor Texas Shell Co. 180 Boxes Lot 2970 Ame. Rx Susan Cavalieri FL Wholesaler 182 Boxes Lot 2970 Premier Medical Group GA Shell Co. 460 Boxes Lot 1091 812 Boxes Lot 2970 CSG TN Wholesaler 812 Boxes Lot 2970 Dialysist West AZ Wholesaler 1056 Boxes, Lot 2970 Dialysist West AZ Wholesaler 461 Boxes, Lot 1091 Amerisource. Bergen KY Distribution Center 1517 Boxes CVS, NY Timothy Fagan CSG TN Wholesaler 460 Boxes Lot 1091

Example 3: Tamiflu Fighting Fake Flu Pills

What can be done? Federal Regulation of Wholesale Distributors: Prescription Drug Marketing Act (PDMA) of 1987, Prescription Drug Amendments (PDA) of 1992 Banned the Sale of Drug Samples and Drug Coupons Banned Reimportation (limited exceptions) Set Requirements for Sample Distribution and Storage Required State Licensing of Wholesale Distributors Required Identity Statements for Sales (pedigree) by Unauthorized Distributors of Record

What can be done? State Licensing of Wholesale Distributors: State Boards of Pharmacy Renewal Schedule: One to Two years Out-of-State Wholesale Distributors Regulatory Challenges Limited Board of Pharmacy Resources Lack of Uniformity of States’ Regulation Lack of Communication Between Regulators

Indiana’s New WDD Law Increases penalties for counterfeiting Rx drugs and distributing contraband drugs Creates rigorous licensing requirements including mandatory accreditation for all wholesalers Determines a “Normal Chain of Distribution” (NCOD) Requires pedigree for all drugs distributed outside of NCOD

Indiana’s New WDD Law Mandatory National Accreditation VAWD® or other Board Approved Accreditation will ensure compliance with relevant state and federal laws Newly issued licensees must obtain Accreditation prior to issuance Existing license holders must obtain prior to next license renewal ( 9/30/06) Negligible fiscal and operational impact on state