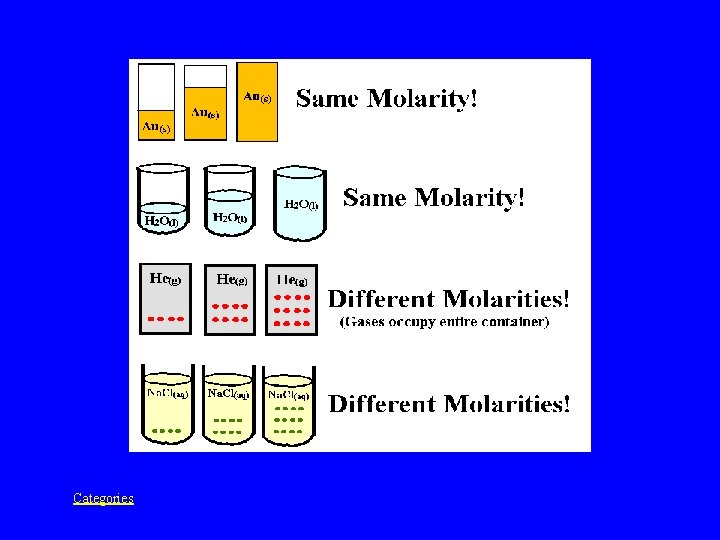
Could you put an image here Categories Whats


















































- Slides: 54

Could you put an image here? Categories

What’s the Word? Weight For It 1023 Concentrated Solutions Toxicity 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

What's the Word? Prompt 100 Points This word is a gas, liquid, or solid that dissolves when mixed with another substance that is usually water. Categories

What's the Word? Response 100 Points What is a solute? Categories

What's the Word? Prompt 200 Points This substance is present in the greatest quantity in a solution. It is usually water, but almost always a liquid. Categories

What's the Word? Response 200 Points What is a solvent? Categories

What's the Word? Prompt 300 Points These substances dissolve completely. Categories

What's the Word? Response 300 Points What is soluble? Categories

What's the Word? Prompt 400 Points This is the sum of the atomic masses (in grams) of a chemical formula. Categories

What's the Word? Response 400 Points What is molar mass? Categories

What's the Word? Prompt 500 Points This chemical unit is the amount of moles of solute per liter of solvent. Categories

What's the Word? Response 500 Points What is Molarity? Categories

Weight For It Prompt 100 Points This is the mass of 1 mole of H 2 O. Categories

Weight For It Response 100 Points What is 18 g? H 2 O = (1 x 2) + (16 x 1) = 18 g Categories

Weight For It Prompt 200 Points This is the mass of 1 mole of C 6 H 12 O 6. Categories

Weight For It Response 200 Points What is 180 g? C 6 H 12 O 6 = (12 x 6) + (1 x 12) + (16 x 6) = 180 g Categories

Weight For It Prompt 300 Points Which is heavier: 1 mole of Na. Cl or 1 mole of KCl? Categories

Weight For It Response 300 Points What is KCl? Na. Cl = 23 + 35 = 58 g KCl = 39 + 35 = 74 g Categories

Weight For It Prompt 400 Points How many grams would 2 moles of Li. Cl weigh? Categories

Weight For It Response 400 Points What is 84 g? Li. Cl = (7 + 35) x 2 = 84 g Categories

Weight For It Prompt 500 Points Would 1 mole of Ca. Cl 2 or 2 moles of HF weigh more? Categories

Weight For It Response 500 Points What is Ca. Cl 2? Ca. Cl 2 = 40 + (35 x 2) = 110 g HF = (1 + 19) x 2 = 40 g Categories

1023 Prompt 100 Points This man discovered the number of molecules in 1 mole is 6. 02 x 1023. Categories

23 10 Response 100 Points Who is Avogadro? Categories

23 10 Prompt 200 Points 1 mole of H 2 O is equal to this many molecules. Categories

23 10 Response 200 Points What is 6. 02 x 1023? Categories

23 10 Prompt 300 Points 0. 5 mole of H 2 O is equal to this many molecules. Categories

23 10 Response 300 Points What is 3. 01 x 1023? Categories

23 10 Prompt 400 Points How many molecules are in 32 g of O 2? Categories

23 10 Response 400 Points What is 6. 02 x 1023? Categories

23 10 Prompt 500 Points If there are 1. 204 x 1024 molecules of NO, how much does NO weigh? Categories

23 10 Response 500 Points What is 60 g? NO = (14 + 16) x 2 = 60 g Categories

Concentrated Solutions Prompt 100 Points If you have 1 mole of Na. Cl in 1 liter of H 2 O, this is the Molarity. Categories

Concentrated Solutions Response 100 Points What is 1 Molarity? 1 mole solute/1 liter of solvent = 1 Molarity Categories

Concentrated Solutions Prompt 200 Points If you have 1 Molarity of KCl, this is how many grams of KCl there are in solution. Categories

Concentrated Solutions Response 200 Points What is 74 g? 1 mole = molar mass Categories

Concentrated Solutions Prompt 300 Points 1. 0 Molarity of Cu. Cl 2 has 133 g in 1 liter of H 2 O. This Molarity is for 13. 3 g of Cu. Cl 2 in 1 liter of H 2 O. Categories

Concentrated Solutions Response 300 Points What is 0. 1 Molarity? In 1 liter, move the decimal to the left. Categories

Concentrated Solutions Prompt 400 Points 1. 0 Molarity of KCl has 74 g in 1 liter of H 2 O. This molarity is for 7. 4 g of KCl in 0. 1 liter of H 2 O. Categories

Concentrated Solutions Response 400 Points What is 1. 0 Molarity? Pretend it’s in 1 liter, move the decimal to the left. If it’s in 0. 1 liter (or 100 m. L), move the decimal to the right. Categories

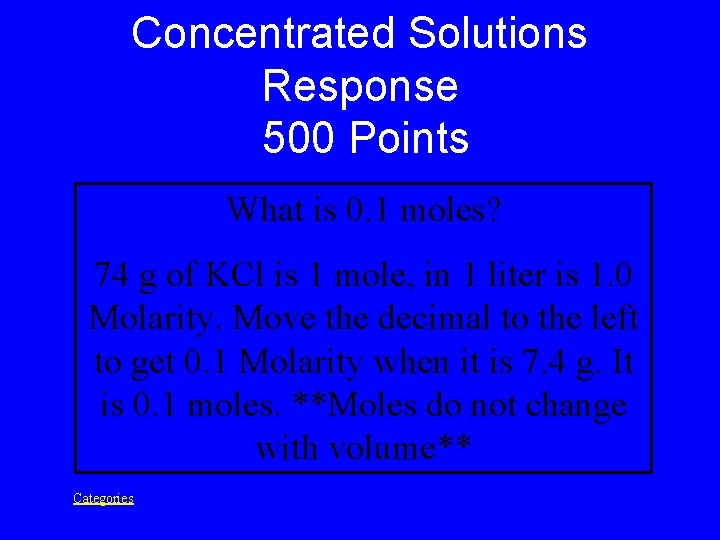
Concentrated Solutions Prompt 500 Points 1. 0 Molarity of KCl has 74 g in 1 liter of H 2 O. This number of moles is for 7. 4 g of KCl in 0. 1 liter of H 2 O. Categories

Concentrated Solutions Response 500 Points What is 0. 1 moles? 74 g of KCl is 1 mole, in 1 liter is 1. 0 Molarity. Move the decimal to the left to get 0. 1 Molarity when it is 7. 4 g. It is 0. 1 moles. **Moles do not change with volume** Categories

Toxicity Prompt 100 Points Is water toxic? Categories

Toxicity Response 100 Points What is yes! Everything is toxic. Categories

Toxicity Prompt 200 Points This is the amount of an ingested substance that kills 50% of a test sample. Categories

Toxicity Response 200 Points What is lethal dose (LD 50)? Categories

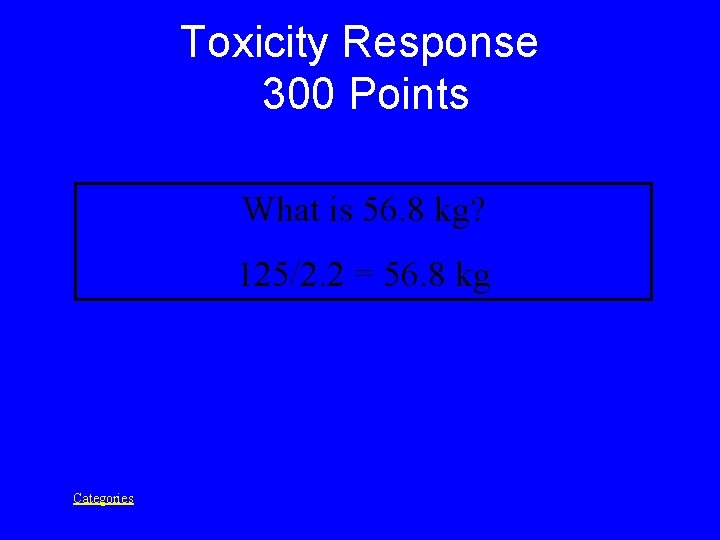
Toxicity Prompt 300 Points If a person weighs 125 pounds, this is that person’s weight in kg. *1 kg = 2. 2 lbs Categories

Toxicity Response 300 Points What is 56. 8 kg? 125/2. 2 = 56. 8 kg Categories

Toxicity Prompt 400 Points The toxic dose of sugar is 30, 000 mg. If a child weighs 30 pounds, how many mg of sugar is lethal? Categories

Toxicity Response 400 Points What is 409, 090 mg? (30/2. 2) x 30, 000 = 409, 090 mg Categories

Toxicity Prompt 500 Points What is more toxic: 1 mole of mercury or 100 g of mercury? Categories

Toxicity Response 500 Points What is 1 mole? 1 mole = 200 g **Comparing LD 50 of different chemicals, the lowest is the most toxic. If it’s the same chemical, the more you have, the more toxic it is. Categories

Put some closing remark here Categories

The Daily Double Categories