CostEffectiveness Analysis of Raltegravir in Treatment Experienced HIV



![Quality of Life Inputs Simpson KN et al (2004) [Base case] ØWeights by CD Quality of Life Inputs Simpson KN et al (2004) [Base case] ØWeights by CD](https://slidetodoc.com/presentation_image_h/a2c136483e227b0e265f08f9faf58cef/image-11.jpg)



- Slides: 19

Cost-Effectiveness Analysis of Raltegravir in Treatment. Experienced HIV Patients in Spain Mohammad A. Chaudhary, Santiago Moreno, Ritesh N. Kumar, Gonzalo Nocea & Elamin Elbasha International AIDS Economics Network Symposium Cuernavava, Mexico, August 1 -2, 2008

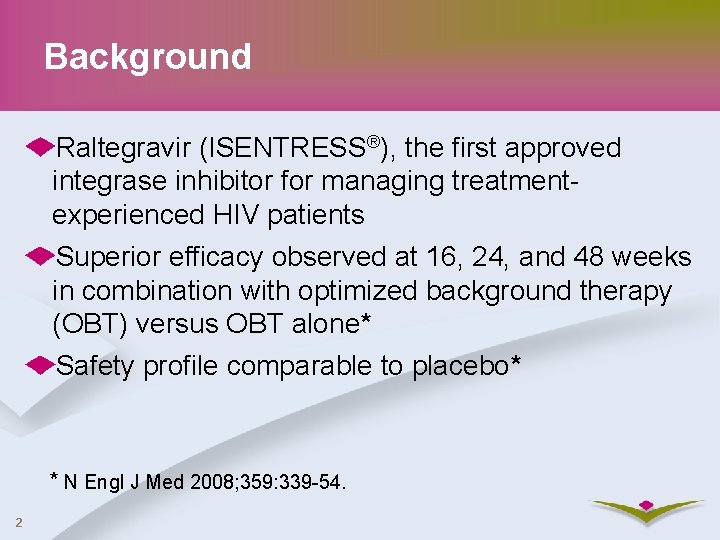
Background Raltegravir (ISENTRESS®), the first approved integrase inhibitor for managing treatmentexperienced HIV patients Superior efficacy observed at 16, 24, and 48 weeks in combination with optimized background therapy (OBT) versus OBT alone* Safety profile comparable to placebo* * N Engl J Med 2008; 359: 339 -54. 2

Objective In light of proven efficacy and safety, the current study evaluates the economic benefits of raltegravir through a cost-effectiveness model Cost-effectiveness is determined by dividing the incremental costs with the drug by the qualityadjusted life years (QALYs) gained by using the drug. 3

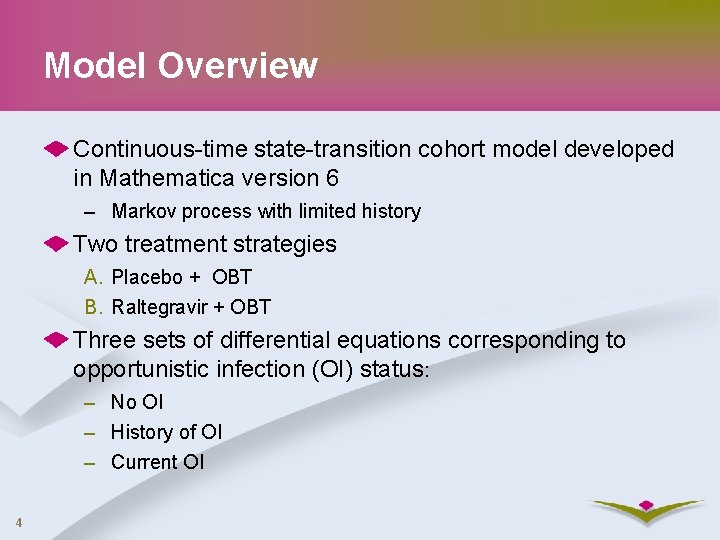
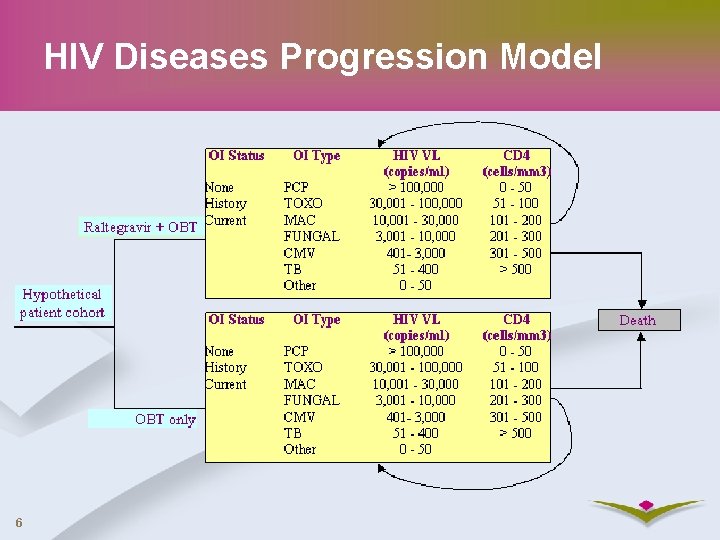
Model Overview Continuous-time state-transition cohort model developed in Mathematica version 6 – Markov process with limited history Two treatment strategies A. Placebo + OBT B. Raltegravir + OBT Three sets of differential equations corresponding to opportunistic infection (OI) status: – No OI – History of OI – Current OI 4

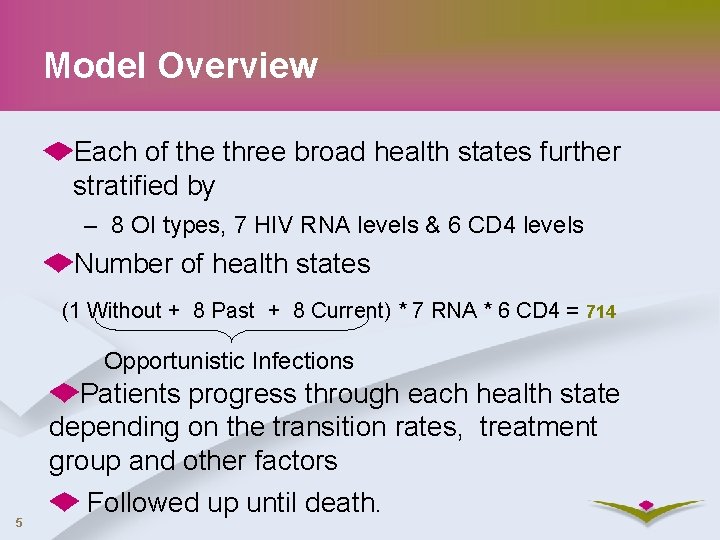
Model Overview Each of the three broad health states further stratified by – 8 OI types, 7 HIV RNA levels & 6 CD 4 levels Number of health states (1 Without + 8 Past + 8 Current) * 7 RNA * 6 CD 4 = 714 Opportunistic Infections 5 Patients progress through each health state depending on the transition rates, treatment group and other factors Followed up until death.

HIV Diseases Progression Model 6

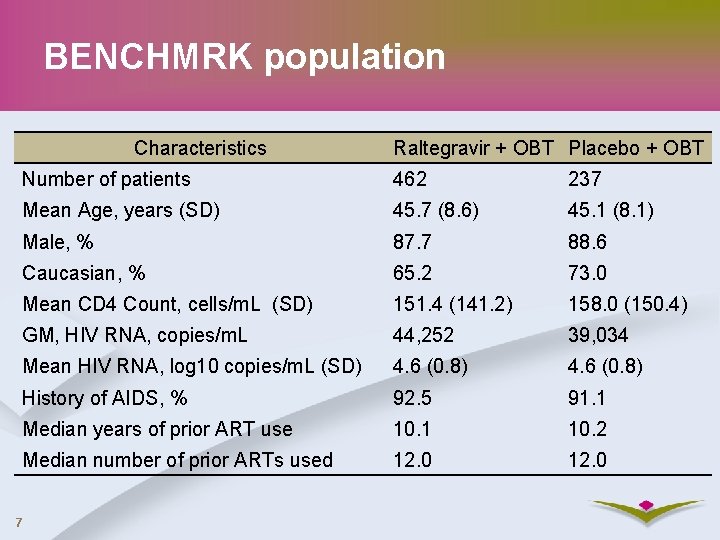
BENCHMRK population Characteristics Raltegravir + OBT Placebo + OBT Number of patients 462 237 Mean Age, years (SD) 45. 7 (8. 6) 45. 1 (8. 1) Male, % 87. 7 88. 6 Caucasian, % 65. 2 73. 0 Mean CD 4 Count, cells/m. L (SD) 151. 4 (141. 2) 158. 0 (150. 4) GM, HIV RNA, copies/m. L 44, 252 39, 034 Mean HIV RNA, log 10 copies/m. L (SD) 4. 6 (0. 8) History of AIDS, % 92. 5 91. 1 Median years of prior ART use 10. 1 10. 2 Median number of prior ARTs used 12. 0 7

Clinical Inputs: HIV RNA Transition intensity matrices for HIV RNA states estimated from BENCHMRK data (Kalbfleisch & Lawless, 1985) Two temporal phases – 0 to 4 weeks – 4 weeks to 48 weeks Annual changes in CD 4 are determined as a function of HIV RNA and CD 4 levels following Euro. SIDA Model assumes treatment effect would remain stable in first 5 years and diminish at a rate of 8% per year thereafter (Phillips et al 2004) 8

Clinical Inputs: Mortality data OIs could have a significant impact on mortality. Three broad categories of mortality rates depending on OI status – Without OI: Spanish male life table data – History of OI: Excess deaths according to CD 4 using Euro. SIDA data, Olsen et al (2005), The PLATO Collaboration (2004) – Current OI: Moore & Chaisson (1996) and Chaisson et al (1998) 9

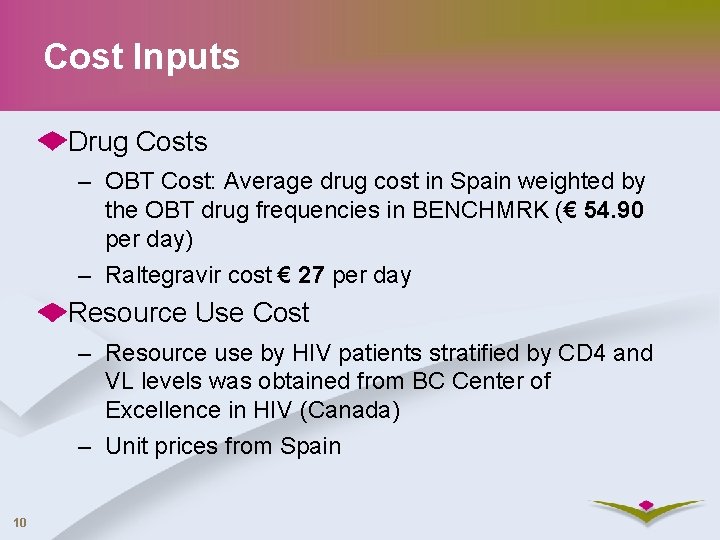
Cost Inputs Drug Costs – OBT Cost: Average drug cost in Spain weighted by the OBT drug frequencies in BENCHMRK (€ 54. 90 per day) – Raltegravir cost € 27 per day Resource Use Cost – Resource use by HIV patients stratified by CD 4 and VL levels was obtained from BC Center of Excellence in HIV (Canada) – Unit prices from Spain 10
![Quality of Life Inputs Simpson KN et al 2004 Base case ØWeights by CD Quality of Life Inputs Simpson KN et al (2004) [Base case] ØWeights by CD](https://slidetodoc.com/presentation_image_h/a2c136483e227b0e265f08f9faf58cef/image-11.jpg)
Quality of Life Inputs Simpson KN et al (2004) [Base case] ØWeights by CD 4 and VL strata ØEQ-5 D, 21000 patients, recent Stavem K et al (2005) [Sensitivity analysis] ØSmall sample, recent, weights by CD 4 only Schackman et al. (2002) and Freedberg (1998)). ØLarge sample, weights CD 4 strata and by OI, history of OI, and no OI history [Sensitivity analysis] 11

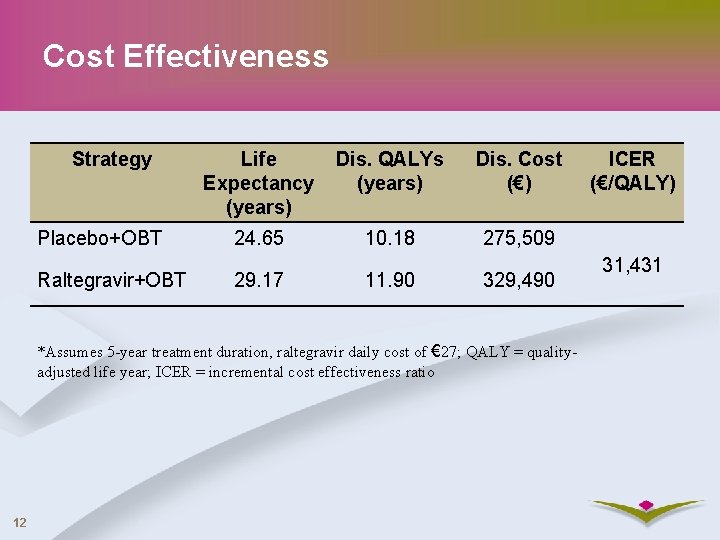
Cost Effectiveness Strategy Placebo+OBT Raltegravir+OBT Life Expectancy (years) Dis. QALYs (years) Dis. Cost (€) 24. 65 10. 18 275, 509 29. 17 11. 90 329, 490 *Assumes 5 -year treatment duration, raltegravir daily cost of € 27; QALY = qualityadjusted life year; ICER = incremental cost effectiveness ratio 12 ICER (€/QALY) 31, 431

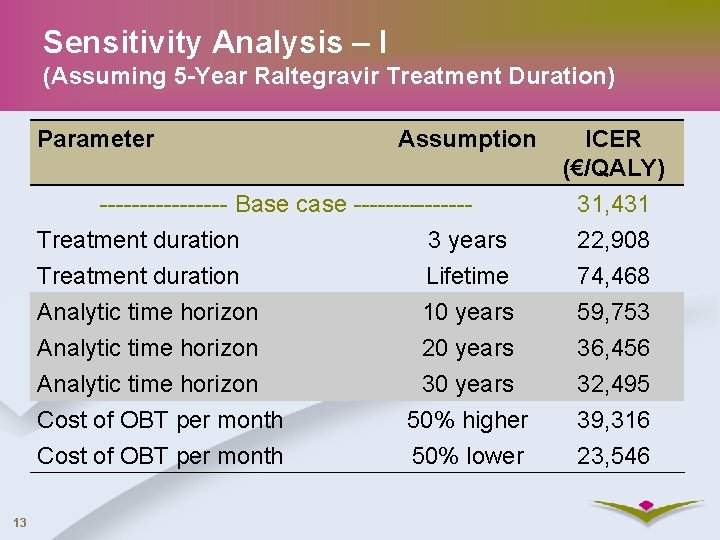
Sensitivity Analysis – I (Assuming 5 -Year Raltegravir Treatment Duration) Parameter Assumption -------- Base case -------Treatment duration 3 years Treatment duration Lifetime Analytic time horizon 10 years Analytic time horizon 20 years Analytic time horizon 30 years Cost of OBT per month 50% higher Cost of OBT per month 50% lower 13 ICER (€/QALY) 31, 431 22, 908 74, 468 59, 753 36, 456 32, 495 39, 316 23, 546

Sensitivity Analysis - II (Assuming 5 -Year Raltegravir Treatment Duration) Parameter Assumption ICER (€/QALY) Cost of raltegravir per month 20% higher 36, 183 Cost of raltegravir per month 20% lower 26, 678 Discount rate/ year: costs & benefits None 21, 325 Discount rate/ year: costs & benefits 3% 23, 414 Incidence of OI per month None 30, 938 Incidence of OI per month Pre c. ART 32, 522 14 *Base case used pre c. CRT OI rates adjusted by data from CASCADE collaboration

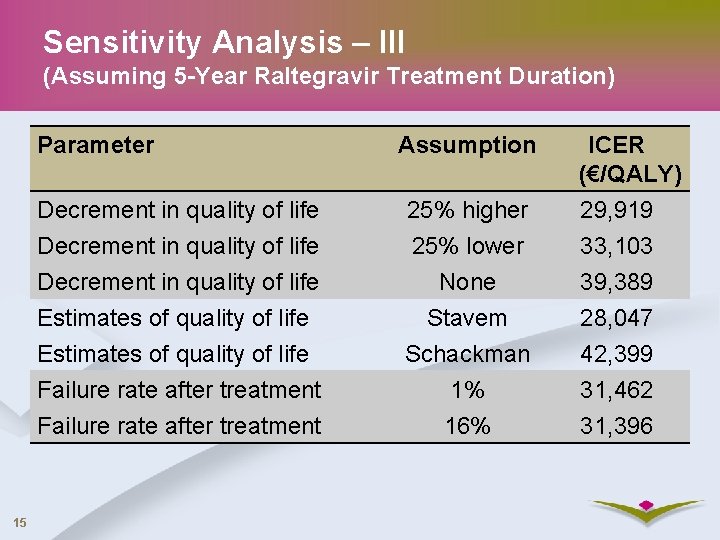
Sensitivity Analysis – III (Assuming 5 -Year Raltegravir Treatment Duration) 15 Parameter Assumption ICER (€/QALY) Decrement in quality of life Estimates of quality of life Failure rate after treatment 25% higher 25% lower None Stavem Schackman 1% 16% 29, 919 33, 103 39, 389 28, 047 42, 399 31, 462 31, 396

Summary Long-term outcomes of raltegravir therapy projected using a cohort state-transition model Model suggests raltegravir provides substantial clinical benefits (e. g. , longer life expectancy) Based on model, raltegravir is cost-effective when added to OBT Results are however sensitive to – Treatment duration – Quality of life weights – Analytical time horizon ICER also sensitive to cost of OBT and raltegravir still raltegravir cost effective 16

Limitations A model is an abstraction of reality Did not model explicitly – Regimen changes over time – Patient compliance – Productivity losses Long-term efficacy not known Data limitations - multiple sources – Incidence, duration, and mortality by CD 4 – HIV-related mortality by CD 4 and VL – Quality of life weights 17

References 1. 2. 3. 4. 5. 6. 7. 8. 9. 18 Babiker A, Darbyshire J, Pezzotti P et al. Changes over calendar time in the risk of specific first AIDS-defining events following HIV seroconversion, adjusting for competing risks. Int J Epidemiol 2002; 31: 951 -958. Chaisson RE, Gallant JE, Keruly JC, Moore RD. Impact of opportunistic disease on survival in patients with HIV infection. Aids 1998; 12: 29 -33. Cooper DA et al. , Subgroup and Resistance Analyses of Raltegravir for Resistant HIV -1 Infection, N Engl J Med 2008; 359: 355 -65 Freedberg KA, Scharfstein JA, Seage GR, III et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA 1998; 279: 130 -136. Jackson CH, Multi-state Modeling with R, the msm package, Version 0. 7. 6, The R Foundation for Statistical Computing, Version 2. 7. 0, 2008. Kalbfleisch JD and Lawless JF. The analysis of panel data under a Markov assumption. Journal of the American Statistical Association, 80(392): 863– 871, 1985. Ledergerber B, Lundgren JD, Walker AS et al. Predictors of trend in CD 4 -positive Tcell count and mortality among HIV-1 -infected individuals with virological failure to all three antiretroviral-drug classes (PLATO Collaboration). Lancet 2004; 364: 51 -62. Mocroft A, Ledergerber B, Viard JP et al. Time to virological failure of 3 classes of antiretrovirals after initiation of highly active antiretroviral therapy: results from the Euro. SIDA study group. J Infect Dis 2004; 190: 1947 -1956. Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med 1996; 124: 633 -642.

References (Contd. ) 10. 11. 12. 13. 14. 15. 16. 19 Olsen CH, Gatell J, Ledergerber B et al. Risk of AIDS and death at given HIV-RNA and CD 4 cell counts, in relation to specific antiretroviral drugs in the regimen. Aids 2005; 19: 319 -330. Phillips AN, Ledergerber B, Horban A et al. Rate of viral rebound according to specific drugs in the regimen in 2120 patients with HIV suppression. Aids 2004; 18: 1795 -1804. Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Medical Decision Making 2002; 22: 27 -38. Simpson KN, Luo MP, Chumney E, Sun E, Brun S, Ashraf T. Cost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infection. HIV Clin Trials 2004; 5: 294 -304. Stavem K, Froland SS, Hellum KB. Comparison of preference-based utilities of the 15 D, EQ-5 D and SF-6 D in patients with HIV/AIDS. Quality of Life Research 2005; 14: 971 -980. Steigbigel RT et al. , Raltegravir with optimized background therapy for resistant HIV-1 Infection, N Engl J Med 2008; 359: 339 -54. Weinstein MC, Goldie SJ, Losina E et al. Use of genotypic resistance testing to guide HIV therapy: Clinical impact and cost-effectiveness. Annals of Internal Medicine 2001; 134: 440 -450.
 Trackball and thumbwheels
Trackball and thumbwheels Have you ever experienced culture shock
Have you ever experienced culture shock 30 cfr part 46
30 cfr part 46 The best pb and j ever text structure
The best pb and j ever text structure Devers experienced the highlight
Devers experienced the highlight More sports at ericson genre
More sports at ericson genre Less + adjective than
Less + adjective than Strength and weaknesses of subject centered design
Strength and weaknesses of subject centered design Whats linear momentum
Whats linear momentum A 23 year old male experienced severe head trauma
A 23 year old male experienced severe head trauma Give two pieces of advice
Give two pieces of advice The resistance r experienced by a partially submerged body
The resistance r experienced by a partially submerged body Brett laming
Brett laming Triệu chứng nhiễm hiv
Triệu chứng nhiễm hiv Where did hiv come from
Where did hiv come from Test wiedzy o aids z odpowiedziami
Test wiedzy o aids z odpowiedziami 10 steps of index testing
10 steps of index testing Prognas akreditasi terbaru 2021
Prognas akreditasi terbaru 2021 Phdp in hiv
Phdp in hiv Kuchecheudzwa
Kuchecheudzwa