Corticosteroid Randomisation After Significant Head Injury Global Health

Corticosteroid Randomisation After Significant Head Injury

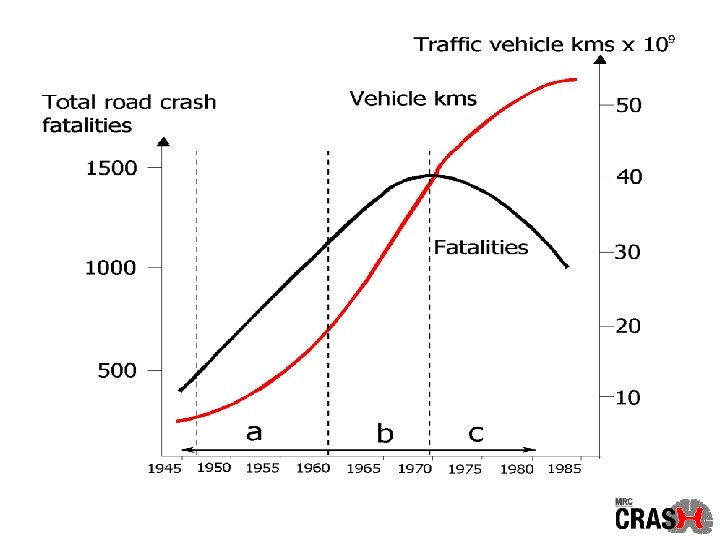
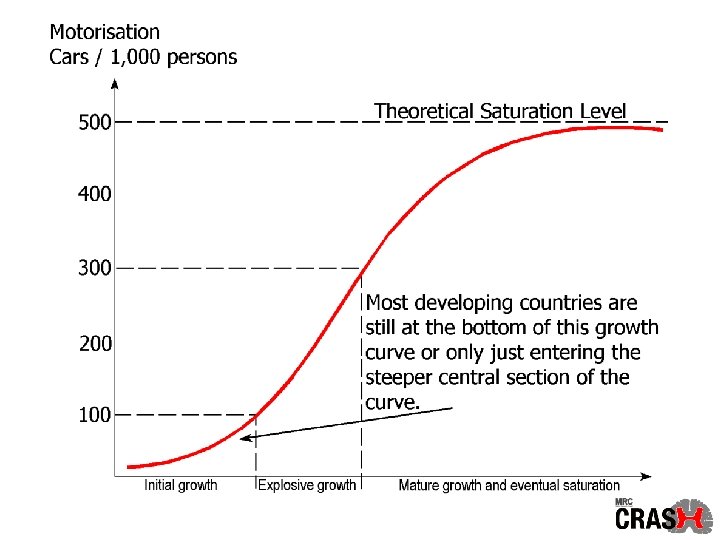
Global Health Statistics In 1990, road traffic crashes caused 5, 563, 000 intracranial injuries worldwide Murray CJL, Lopez AD. Global health statistics. Boston: Harvard University Press, 1996
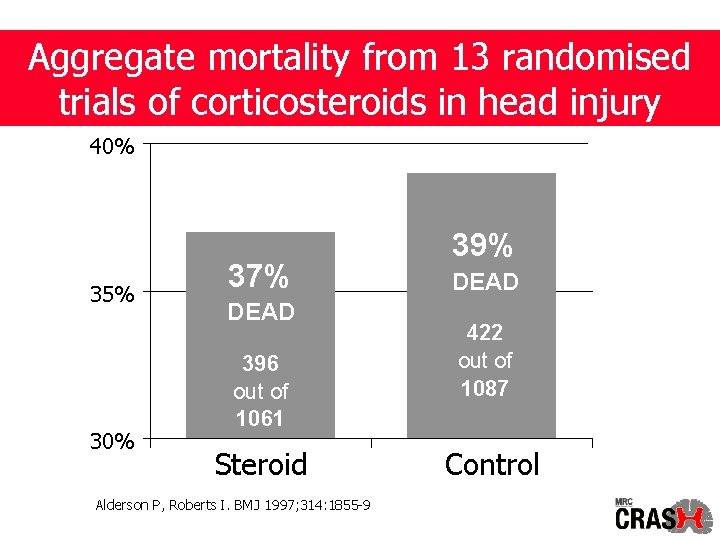
Aggregate mortality from 13 randomised trials of corticosteroids in head injury 40% 35% 30% 37% DEAD 396 out of 1061 Steroid Alderson P, Roberts I. BMJ 1997; 314: 1855 -9 39% DEAD 422 out of 1087 Control
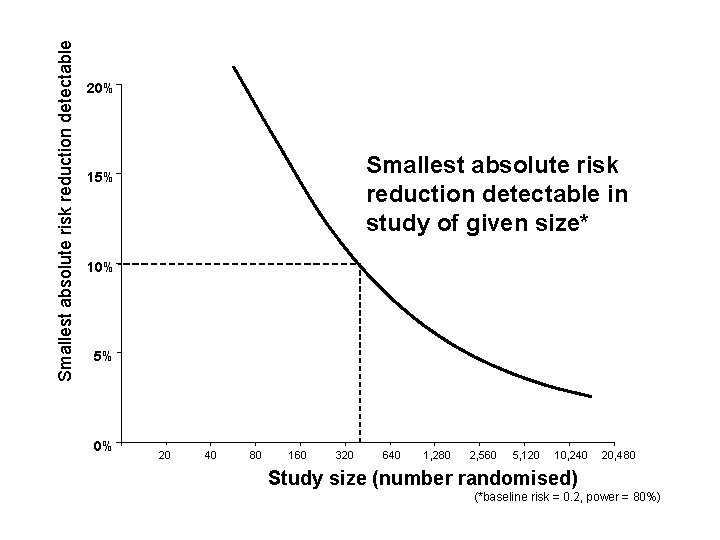
Smallest absolute risk reduction detectable 20% Smallest absolute risk reduction detectable in study of given size* 15% 10% 5% 0% 20 40 80 160 320 640 1, 280 2, 560 5, 120 10, 240 20, 480 Study size (number randomised) (*baseline risk = 0. 2, power = 80%)
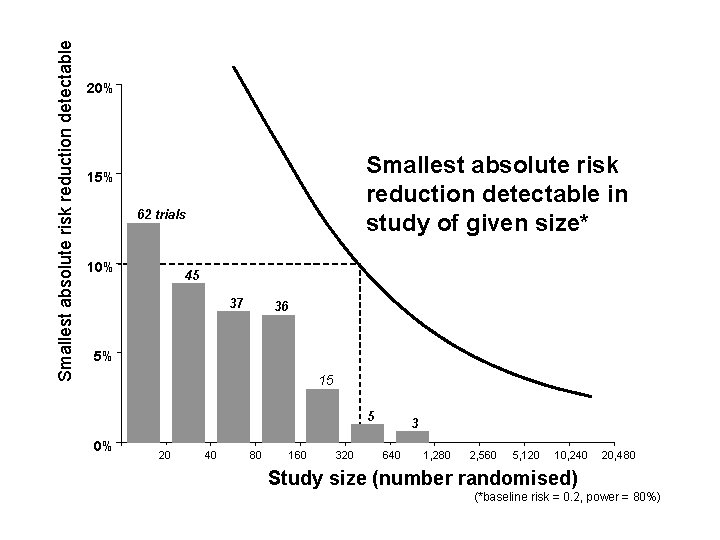
Smallest absolute risk reduction detectable 20% Smallest absolute risk reduction detectable in study of given size* 15% 62 trials 10% 45 37 36 5% 15 5 0% 20 40 80 160 320 3 640 1, 280 2, 560 5, 120 10, 240 20, 480 Study size (number randomised) (*baseline risk = 0. 2, power = 80%)

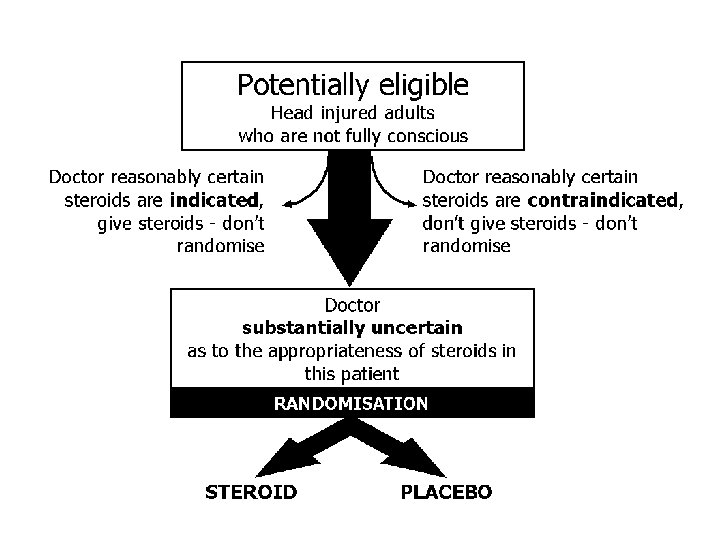
A large simple placebo controlled trial, among adults with head injury and impaired consciousness, of the effects of a 48 -hour infusion of corticosteroids on death and neurological disability
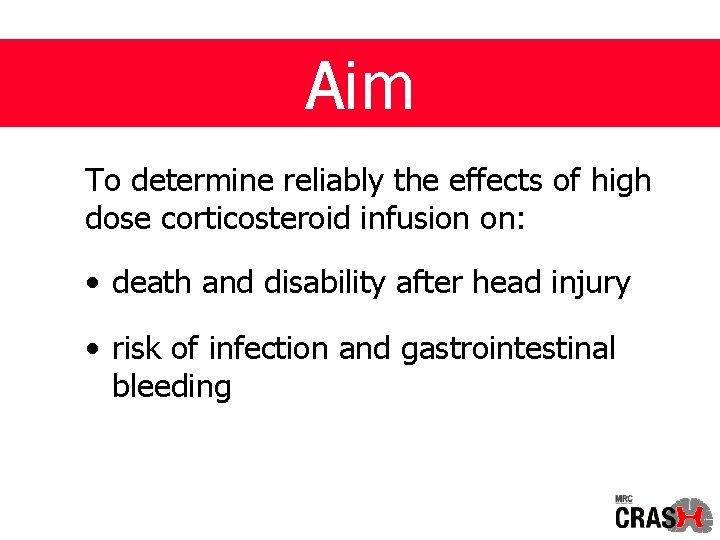
Aim To determine reliably the effects of high dose corticosteroid infusion on: • death and disability after head injury • risk of infection and gastrointestinal bleeding

Randomisation Take next numbered treatment pack Fax entry form to Co-ordinating Centre - Pack number - Patient details - Hours since injury - GCS - Pupil reactiveness
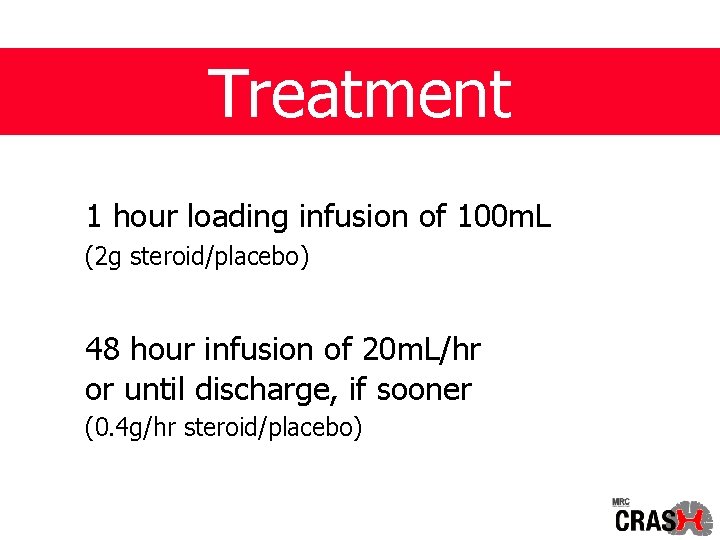
Treatment 1 hour loading infusion of 100 m. L (2 g steroid/placebo) 48 hour infusion of 20 m. L/hr or until discharge, if sooner (0. 4 g/hr steroid/placebo)

Adverse events Serious and unexpected adverse events suspected to be related to trial medicine Telephone UK randomisation service and ask for “adverse events”

Unblinding If care depends importantly on knowing whether patient received steroid or not Telephone UK randomisation service and ask for “unblinding”

Follow-up No extra tests Single-sided outcome form completed at discharge, death in hospital, or 14 days Short interview or postal questionnaire sent at six months



Trial progress • Started - April 1999 • Estimated end - March 2005 • Target - 20, 000 patients • Recruitment to date - 2, 420 patients
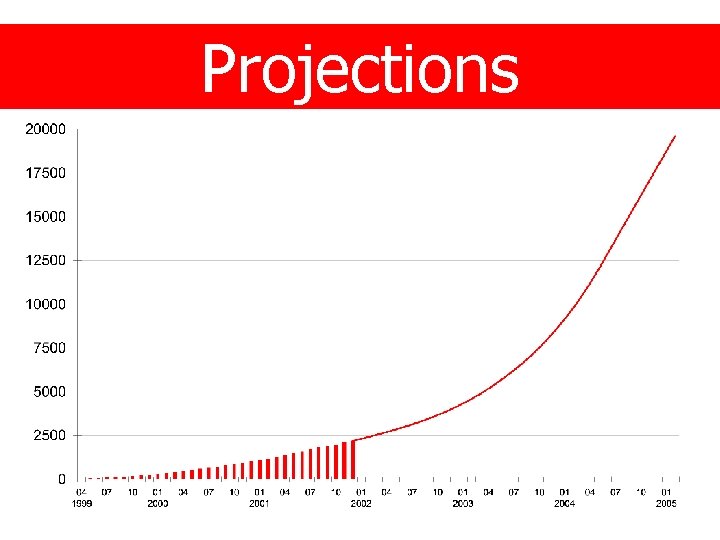
Projections
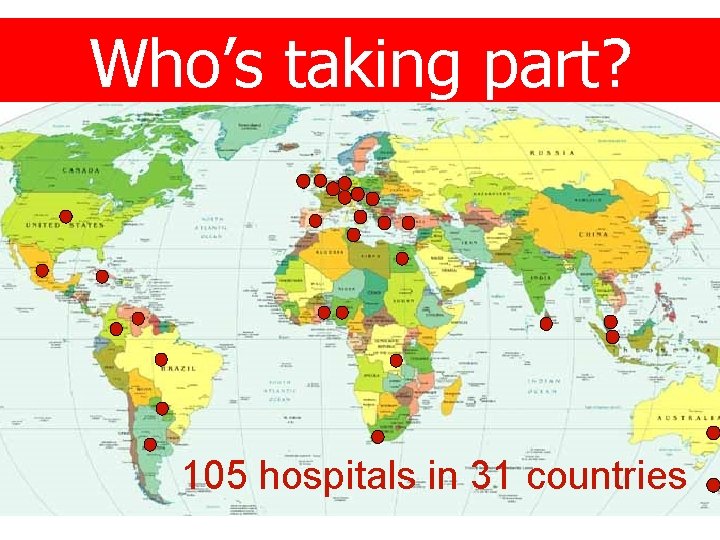
Who’s taking part? 105 hospitals in 31 countries
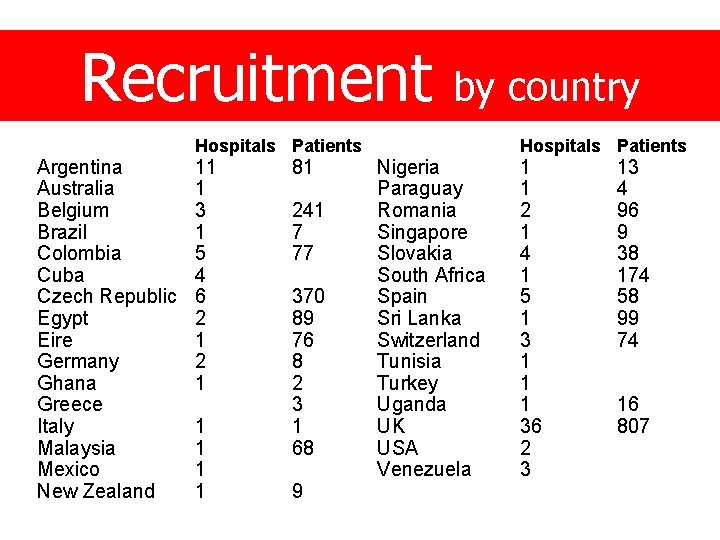
Recruitment Argentina Australia Belgium Brazil Colombia Cuba Czech Republic Egypt Eire Germany Ghana Greece Italy Malaysia Mexico New Zealand Hospitals Patients 11 1 3 1 5 4 6 2 1 1 1 81 241 7 77 370 89 76 8 2 3 1 68 9 by country Nigeria Paraguay Romania Singapore Slovakia South Africa Spain Sri Lanka Switzerland Tunisia Turkey Uganda UK USA Venezuela Hospitals Patients 1 1 2 1 4 1 5 1 3 1 1 1 36 2 3 13 4 96 9 38 174 58 99 74 16 807

Recruitment rates Hospital Research Institute for Special Surgery and Trauma Centre Hospitalier Regional de Namur Spitalul Clinic de Urgenta Bucuresti Tygerberg Academic Hospital Makerere Medical School Mataria Teaching Hospital University Science Malaysia Hope Hospital University Hospital of Zurich Clinica Las Americas St James's University Hospital Birmingham Heartlands Hospital Universitario Virgen del Rocio Suez Canal University Hospital San Bernardo Batticaloa General Hospital, Teaching Ns. P Poprad Country Czech Republic Belgium Romania South Africa Uganda Egypt Malaysia UK Switzerland Colombia Eire UK Spain Egypt Argentina Sri Lanka Slovakia Patients/month 9. 6 8. 2 7. 7 7. 3 5. 5 4. 4 3. 9 3. 2 3. 0 2. 8 2. 4 2. 3 2. 2

Some results • Pilot phase 13/4/1999 to 21/12/2000 • 1, 000 patients enrolled • Early outcome (14 days) 99% complete • Six month outcome 93% complete

Age and sex Age group 16 – 24 25 – 34 35 + n 259 227 514 % (26) (23) (51) Sex Male Female 781 219 (78) (22)
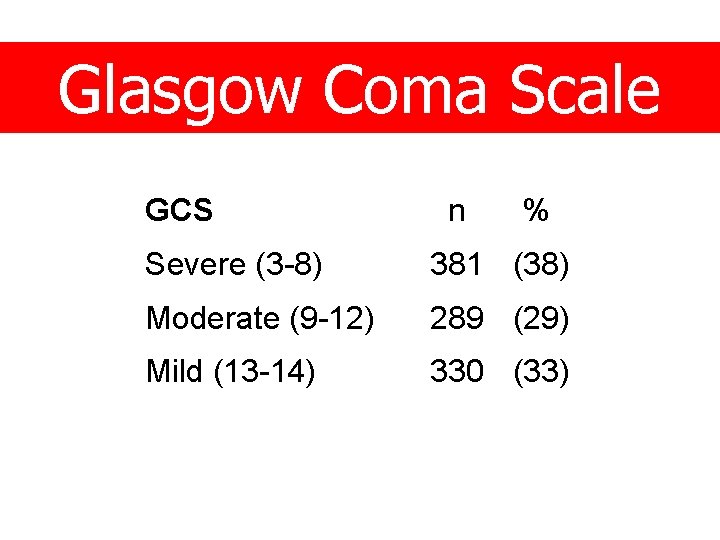
Glasgow Coma Scale GCS n % Severe (3 -8) 381 (38) Moderate (9 -12) 289 (29) Mild (13 -14) 330 (33)
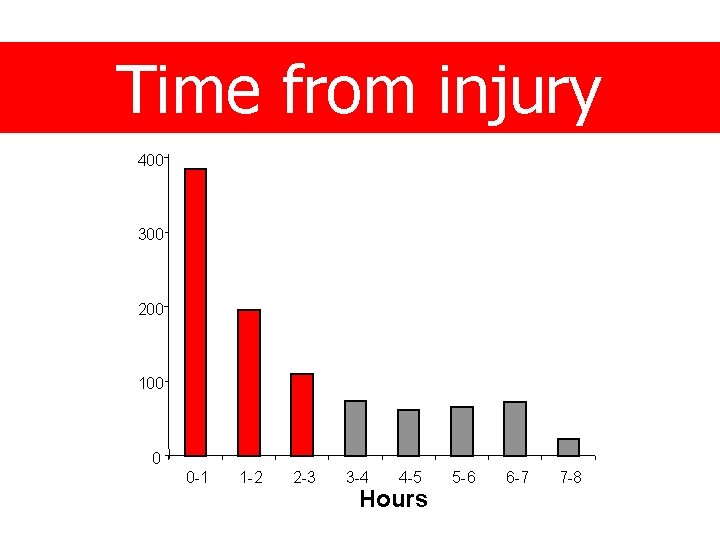
Time from injury 400 300 200 100 0 0 -1 1 -2 2 -3 3 -4 4 -5 Hours 5 -6 6 -7 7 -8
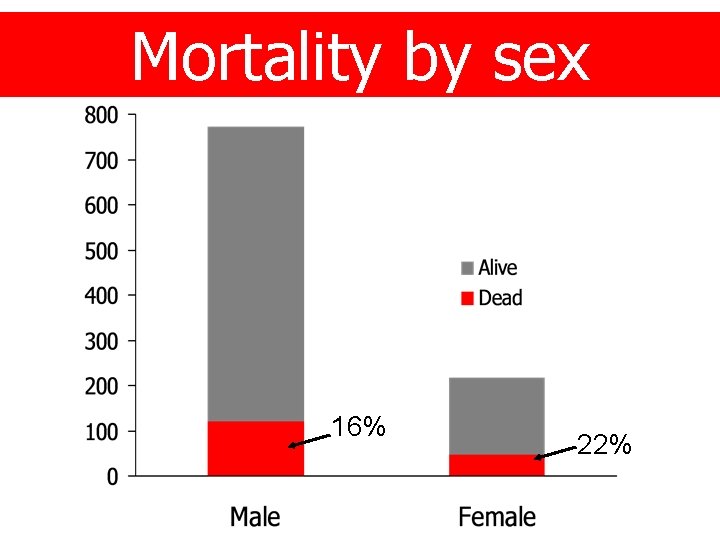
Mortality by sex 16% 22%
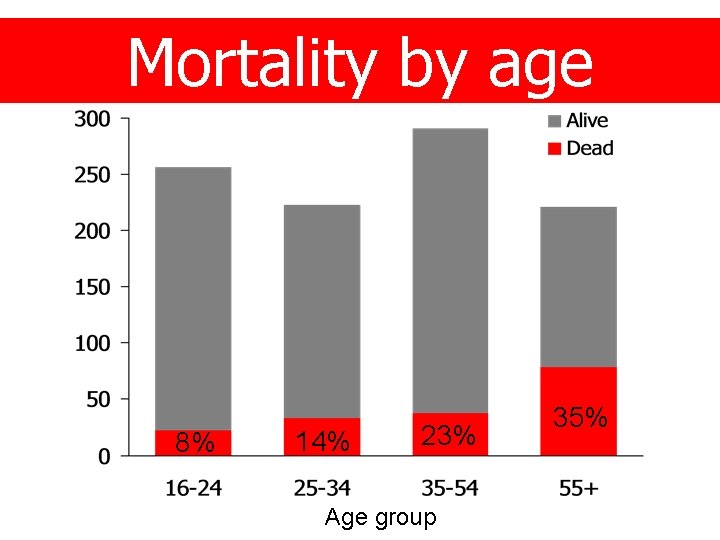
Mortality by age 8% 14% 23% Age group 35%
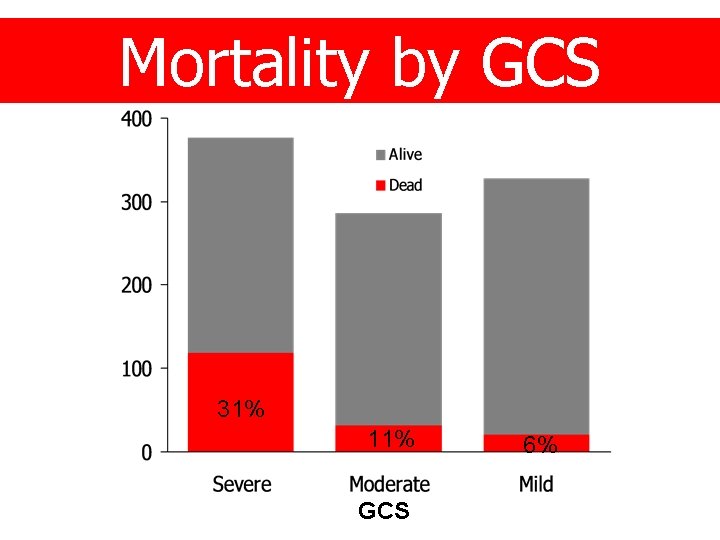
Mortality by GCS 31% 11% GCS 6%
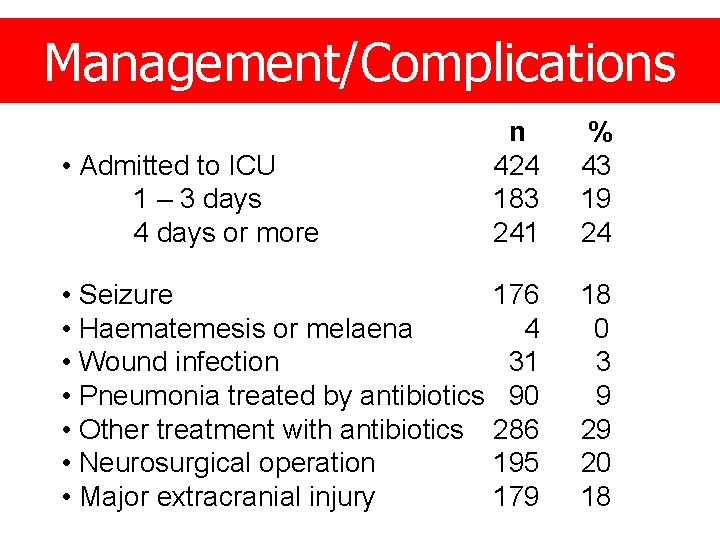
Management/Complications n 424 183 241 % 43 19 24 • Seizure 176 • Haematemesis or melaena 4 • Wound infection 31 • Pneumonia treated by antibiotics 90 • Other treatment with antibiotics 286 • Neurosurgical operation 195 • Major extracranial injury 179 18 0 3 9 29 20 18 • Admitted to ICU 1 – 3 days 4 days or more

Conclusions to date • The MRC CRASH Trial is working well in both university and general hospitals • The assumed mortality rate is appropriate • Large numbers can be successfully followed-up

www. crash. lshtm. ac. uk CRASH Co-ordinating Centre 49 -51 Bedford Square, London WC 1 B 3 DP Tel: +44 (0)20 7299 4684 Fax: +44 (0)20 7299 4663 CRASH@LSHTM. ac. uk
- Slides: 32