CORROSION TYPES CHAPTER 4 6 SLECTIVE LEACHING Ass







- Slides: 9

CORROSION TYPES CHAPTER 4 6) SLECTIVE LEACHING Ass. Professor SAHEB M. MAHDI

6 ) SELECTIVE LEACHING (Dealloying, Parting) Corrosion in which one constituent of an alloy is preferentially removed, leaving behind an altered (weakened) residual structure, Can occur in several systems.

►Dezincification : It is a form of corrosion in which zinc is selectively attacked in zinc- containing alloys, like brasses. De-alloying and selective leaching are broader terms which refer to the corrosion of one or more constituent of a solid solution alloy. Dezincification is a form of de-alloying. As the phenomenon was first observed in brass in which zinc separated by dissolution from copper. All Cu-Zn alloys (Brasses) containing > 15% Zn are susceptible e. g. common yellow brass. . . 30 Zn 70 Cu, dezincifies to red copper-rich structure. Dezincification can be in two types , (1)uniform type , like the potable water inside. In the uniform the active area is leached out over a broad area of the surface and it is not localized to certain point of the surface. Uniform dezincification. . . usually found in high brasses (high[Zn]), acid environments;

(2)plug type, like (boiler water inside, combustion gases outside. the plug type of attack is localized, at a certain point on the surface and surrounding area remains unaffected. Plug-type dezincification. . . usually found in low brasses, alkaline, neutral or slightly acid environments.

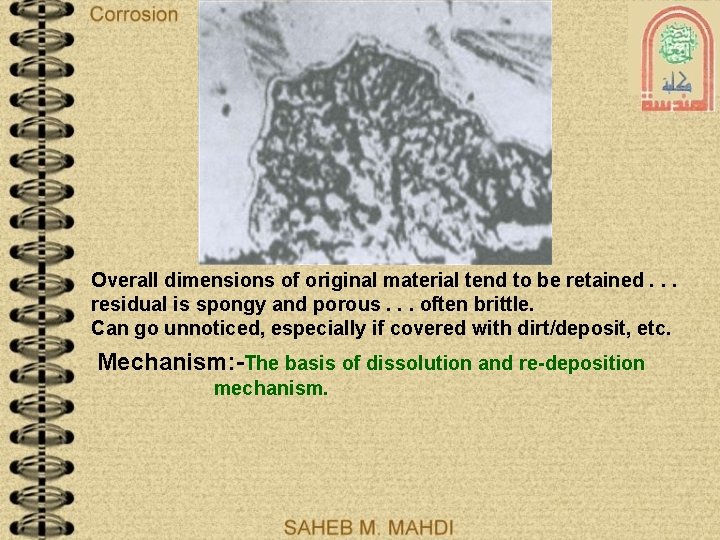
Overall dimensions of original material tend to be retained. . . residual is spongy and porous. . . often brittle. Can go unnoticed, especially if covered with dirt/deposit, etc. Mechanism: -The basis of dissolution and re-deposition mechanism.

)1) Zn atoms leave lattice sites. . . “are leached into the environment selectively’’ Zn → Zn+2 + 2 e 2 H+ + 2 e → H 2 Cu. Cl + e → Cu + Cl- (I) dissolution M → M+n + ne 2 H+ + 2 e → H 2 Cu → Cu+2 + 2 e (II) dissolution 2 H+ + 2 e → H 2 Cu+2 + Zn → Cu + Zn+2 (III) plating Step I Dissolution of Cu and Zn ( eq. I & II ). Step II Zinc stays in solution. Step III Copper plates back ( eq. III ). (2) Generally accepted. . . -brass dissolves; -Zn stays in solution; -Cu re-deposits.

possibility for local anode-cathode couples. . Cu deposits accelerate attack. dezincified areas generally 90 -95% Cu; some Cu 2 O/Cu. O present if O 2 in the environment. Prevention: -Make environment less aggressive (e. g. , reduce O 2 content); -Cathodically protect; -Use a better alloy (common cure - above not usually feasible). . . - “red” brass (<15% Zn) almost immune - Admiralty Brass. . ( 70 Cu, 29 Zn, 1 Sn ). - arsenical Admiralty. . ( 70 Cu, 29 Zn, 1 Sn, 0. 04 As ). (Sn and Sn-As in deposited films hinder redeposition of Cu). -For very corrosive environments likely to provoke dezincification, or for critical components, use cupronickels ( 70 -90 Cu , 30 -10 Ni ).

►Graphitization : -) misnomer. . . graphitization is the breakdown of Pearlite to ferrite + C at high temperature). Grey cast iron is the cheapest engineering metal 2 -4% C, 1 -3% Si. Hard, brittle, easily cast; carbon present as microscopic flakes of matrix graphite within microstructure. In some environments (notably mild, aqueous soils affecting buried pipe) the Fe leaches out slowly and leaves graphite matrix behind. . appears graphitic. . . soft. . . can be cut with a knife. Pores usually filled with rust. Original dimensions are retained.

Micrograph of symmetrical envelopes of graphitically-corroded iron surrounding flakes of graphite.