Corrosion Part 3 Corrosion Protection Methods Corrosion Monitoring






















- Slides: 30

Corrosion Part 3 Corrosion Protection Methods Corrosion Monitoring Devices Corrosion Inspection Procedures Environmental Factors 1

Corrosion Protection Methods Slides 3 to 11 2

Maintaining p. H � Keep the p. H of the water between 9 and 11. ◦ This reduces acid or alkaline corrosion to a minimum. �This is usually done by adding sodium hydroxide to neutral water. �Alkaline amines such as cyclohexylamine are added since they are volatile and carry over with the steam to prevent acid corrosion in the condensate return system. 3

Mechanical Deaeration of O 2 �Mechanical Deaeration (removal of dissolved oxygen) �Remove dissolved oxygen from water. ◦ This is done by heating the water to close to 100°C to drive off the dissolved gases in a deaerator. �Oxygen solubility decreases with temperature �This water must not be re-exposed to air or more oxygen will dissolve. 4

Chemical Deaeration of O 2 �Chemical Oxygen Scavengers �If an oxygen scavenger chemical is dissolved in the water it will react with the dissolved oxygen to reduce its concentration. ◦ Sodium sulfite protects boilers. � 2 Na 2 SO 3 + O 2 Na 2 SO 4 �Carbohydrazide is volatile so it carries over with steam to protect condensate. ◦ (N 2 H 3)2 CO + 2 O 2 2 N 2 + 3 H 2 O + CO 2 5

Galvanic Protection – Sacrificial Metals 3. Sacrificial Metals �If a metal higher than iron on the activity series such as zinc is bonded in good electrical contact to the iron, the zinc will corrode first, protecting the iron from corrosion. ◦ This method of corrosion protection is called galvanic protection. �See the video referenced in the next slide 6

Video on Galvanic Cell Video http: //www. youtube. com/watch? v=0 o. Sq. PDD 2 r. MA �Both Zn. SO 4 and Cu. SO 4 are 100% ionized �Zn, more anodic (reactive) than Cu, gives up its electrons to Cu ◦ referring to the Activity Series for metals, Zn is more reactive than Cu �The zinc electrode corrodes over time 7

Corrosion Protection Methods – Corrosion Inhibitors 4. Protective Coatings �If the metal is coated so it is not exposed to water it will resist corrosion. �Aluminum oxide forms a durable oxide coat that protects aluminum from corrosion. �The chromium in stainless steel forms a durable oxide coat offering corrosion protection. �Magnetite (a form of iron oxide) protects iron. �Rust paints work by isolating the metal from water. 8

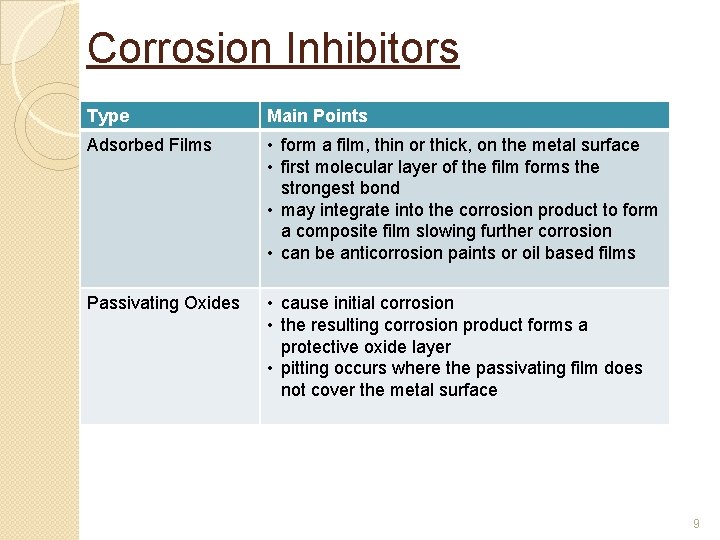
Corrosion Inhibitors Type Main Points Adsorbed Films • form a film, thin or thick, on the metal surface • first molecular layer of the film forms the strongest bond • may integrate into the corrosion product to form a composite film slowing further corrosion • can be anticorrosion paints or oil based films Passivating Oxides • cause initial corrosion • the resulting corrosion product forms a protective oxide layer • pitting occurs where the passivating film does not cover the metal surface 9

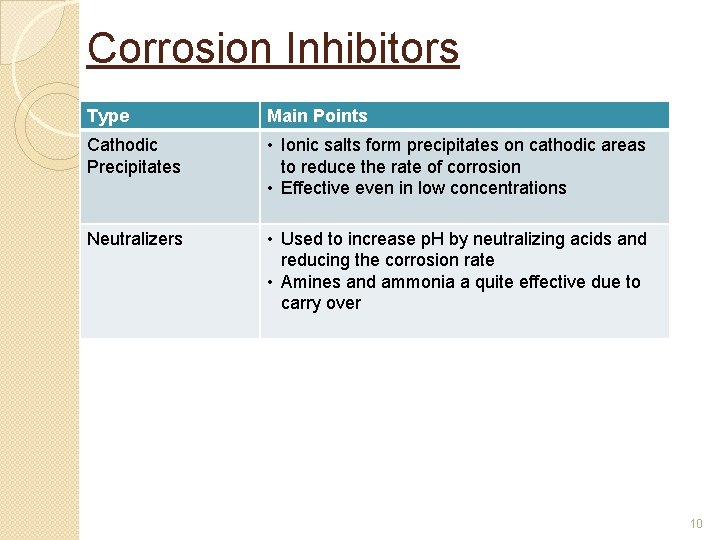
Corrosion Inhibitors Type Main Points Cathodic Precipitates • Ionic salts form precipitates on cathodic areas to reduce the rate of corrosion • Effective even in low concentrations Neutralizers • Used to increase p. H by neutralizing acids and reducing the corrosion rate • Amines and ammonia a quite effective due to carry over 10

Corrosion Protect Methods – Impressed Current 5. Impressed Current �If the iron is connected to the negative terminal of a DC power supply, this will supply electrons to the iron preventing its loss of electrons (oxidation). ◦ A current of about 50 to 100 m. A is required for each square meter of iron to be protected. 11

Corrosion Monitoring Devices �Slides 13 to 17 12

Corrosion Coupons �Corrosion coupons are uniform sized, weighed pieces of metal representative of system metals �Used to determine corrosion in a system over time: ◦ system corrosiveness ◦ material performance ◦ inhibitor effectiveness �Applicable for gases, liquids, particulate flow 13

Electrical Resistance Probes �A conductive metal, exposed to corrosion, will have its surface area reduced, increasing its electrical resistance �Using a length of wire in a corrosive environment an electrical resistance probe can measure the change in resistance of the wire over time �This is used to determine the corrosion rate �Applicable for gases, liquids, particulate flow 14

Galvanic Probes �Operates on the principle of the galvanic cell ◦ Electrodes immerse in the fluid ◦ The potential difference generates a current �The current relates to the rate of corrosion �Used in water injection systems to monitor for dissolved oxygen concentrations 15

Hydrogen Probes �Hydrogen probes measure the amount of hydrogen permeating through the metal �Two types mentioned ◦ one design uses pressure of H 2 gas generated by diffuse H atoms as a measure of the rate of corrosion ◦ another uses an electrochemical cell that attaches to the surface of a pipe �current is generated to oxidize surface hydrogen �corrosion rate is proportional to the current produced. 16

Corrosion Inspection Procedures �Slides 19 to 23 17

Direct Observation �All observations need to be properly documented ◦ start with a baseline condition �Include: ◦ ◦ photographs date, time written description assessment of any damage 18

Magnetic Particle Inspection �Used for ferromagnetic metals (can be magnetized) such as carbon steel ◦ magnetize the area of interest ◦ sprinkle on iron powder or filings ◦ filings indicate the location and size of cracks in the metal 19

Dye Penetrant Inspection �Indicates surface flaws only �Low cost method of inspection �Kit includes: ◦ surface cleaner ◦ indicator dye ◦ a developer which draws the dye back out of the surface defect indicating its location 20

Ultrasonic Inspection �Uses ultrasound to detect flaws on or beneath the metal surface ◦ based on sound propagation and reflection �Used to inspect welds 21

Radiography �In essence an “x-ray” of a horizontal cross-section of pipe �x-rays or gamma rays are used �corroded areas show up as dark spots on the “x-ray” film 22

Environmental Factors �Slides 25 to 32 23

Fluid Velocities �Velocity is too fast ◦ wears away protective, anti-corrosion film on metal surface �Velocity is too slow ◦ particles settle out of fluid on the metal surface preventing anti-corrosion chemical treatment �sites of particle accumulation subject to corrosion 24

p. H �p. H < 9 promotes corrosion ◦ in anodic areas low p. H corrodes protective metal oxide films �due to oxidation of iron ◦ in cathodic areas low p. H produces OH− �results in Fe(OH)2 �as acidity increases the rate of corrosion increases 25

Temperature �Corrosion related reactions become more vigorous as temperature increases �warmer areas on a piece of metal become anodic ◦ subject to corrosion 26

Oxygen Content �Corrosion rates increase as oxygen levels around the cathode area increase ◦ electrons travelling from the anodic to cathodic areas combine with oxygen at the cathode’s surface 27

Microorganisms �Can change the amount of oxygen in the water affecting corrosion rates ◦ can increase rate by supplying oxygen to cathodic areas of the system ◦ can form deposits on metal surfaces with other matter �an anodic site develops �results in pitting 28

Dissolved Solids and Gases �Dissolved solids result in: ◦ interference of formation of corrosion inhibitor films ◦ increasing the fluids ability to conduct current which increases corrosion �Dissolved gases result in: ◦ O 2 combines electrons at the cathode ◦ CO 2 H 2 S increases acidity 29

Cooling Water Systems �Due to design and construction these systems are a galvanic cell type of corrosion ◦ cooling water is oxygen rich ◦ cooling water high is dissolved solids ◦ equipment temperature differences produce anodic and cathodic areas ◦ water can become acidic ◦ biological growth can occur 30