Corrosion Intermediate 2 Unit 3c WHAT IS CORROSION








- Slides: 23

Corrosion Intermediate 2 Unit 3(c)

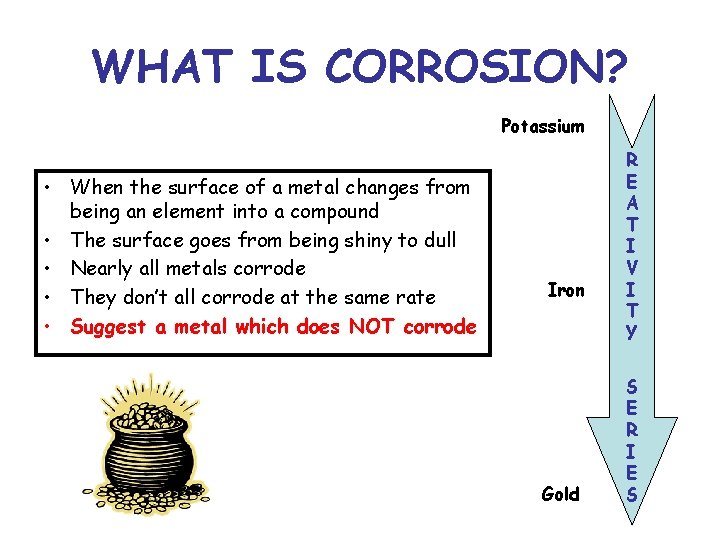
WHAT IS CORROSION? Potassium • When the surface of a metal changes from being an element into a compound • The surface goes from being shiny to dull • Nearly all metals corrode • They don’t all corrode at the same rate • Suggest a metal which does NOT corrode Iron Gold R E A T I V I T Y S E R I E S

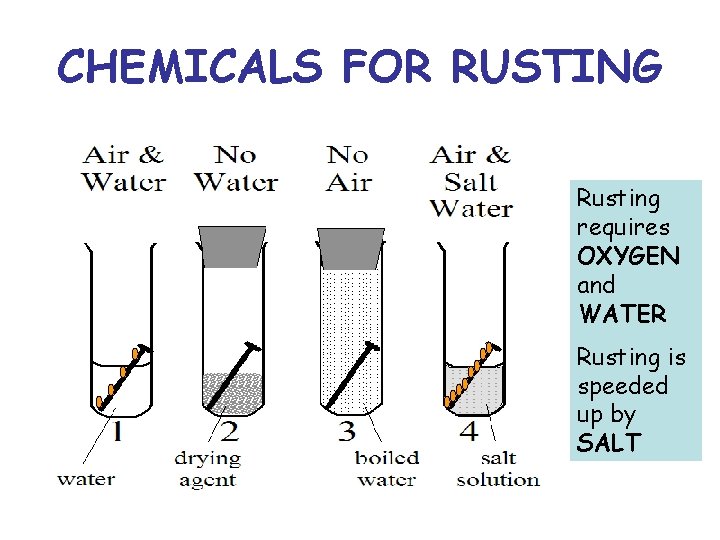
CHEMICALS FOR RUSTING Rusting requires OXYGEN and WATER Rusting is speeded up by SALT

RUSTING • The corrosion of iron is called rusting • Iron is the only metal which rusts • View Rusting practical demo Why did the water rise up the tube? How would the rate of the water rising compare if the wool had been soaked in water instead of acid?

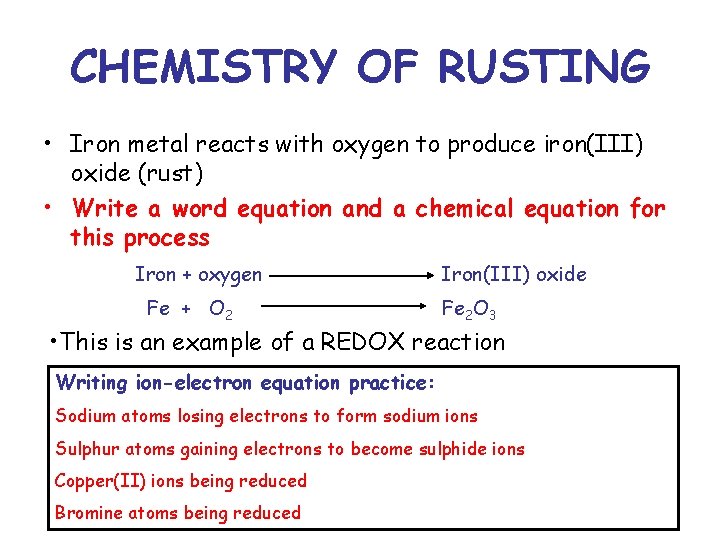
CHEMISTRY OF RUSTING • Iron metal reacts with oxygen to produce iron(III) oxide (rust) • Write a word equation and a chemical equation for this process Iron + oxygen Fe + O 2 Iron(III) oxide Fe 2 O 3 • This is an example of a REDOX reaction Writing ion-electron equation practice: Sodium atoms losing electrons to form sodium ions Sulphur atoms gaining electrons to become sulphide ions Copper(II) ions being reduced Bromine atoms being reduced

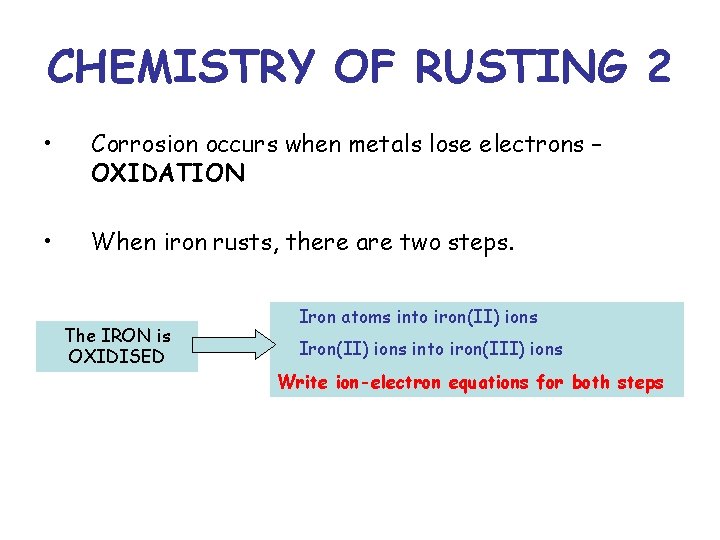
CHEMISTRY OF RUSTING 2 • Corrosion occurs when metals lose electrons – OXIDATION • When iron rusts, there are two steps. The IRON is OXIDISED Iron atoms into iron(II) ions Iron(II) ions into iron(III) ions Write ion-electron equations for both steps

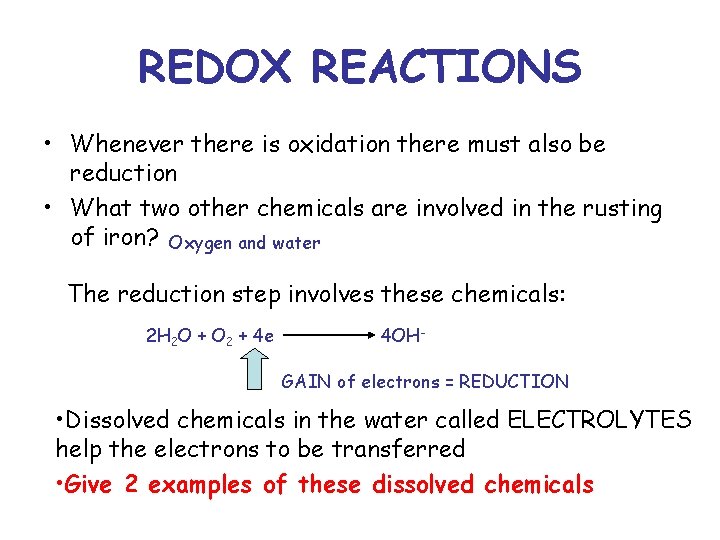
REDOX REACTIONS • Whenever there is oxidation there must also be reduction • What two other chemicals are involved in the rusting of iron? Oxygen and water The reduction step involves these chemicals: 2 H 2 O + O 2 + 4 e 4 OHGAIN of electrons = REDUCTION • Dissolved chemicals in the water called ELECTROLYTES help the electrons to be transferred • Give 2 examples of these dissolved chemicals

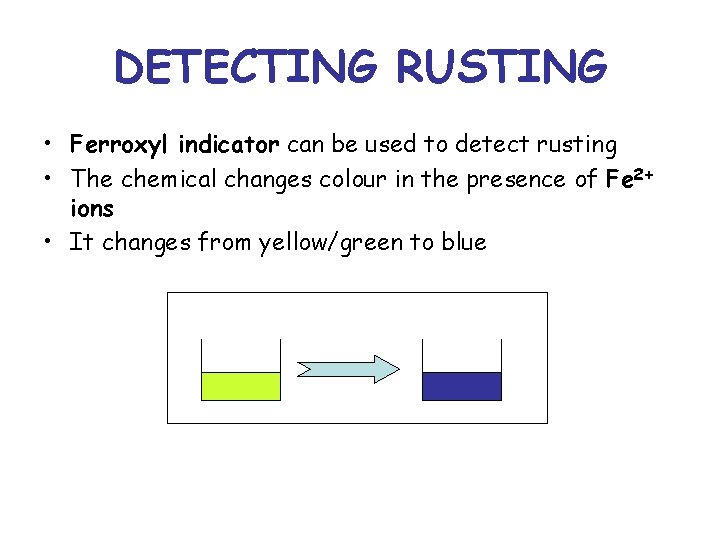
DETECTING RUSTING • Ferroxyl indicator can be used to detect rusting • The chemical changes colour in the presence of Fe 2+ ions • It changes from yellow/green to blue

TUTORIAL QUESTIONS True or false: 1. 2. 3. 4. 5. 6. Corrosion is the rusting of iron p. H indicator should be used to test for rusting Rusting takes place in the presence of oxygen only Rusting will not take place if water is not present Salt is required for rusting to take place Rusting will occur faster in sea water than tap water Problem: Rusting occurs faster in salt solution than in pure water. (a) Write an ion-electron equation for the rusting of iron (b)Why does rusting occur faster in salt solution than pure water? (c) Suggest why rusting also occurs faster in acid solution than in pure water

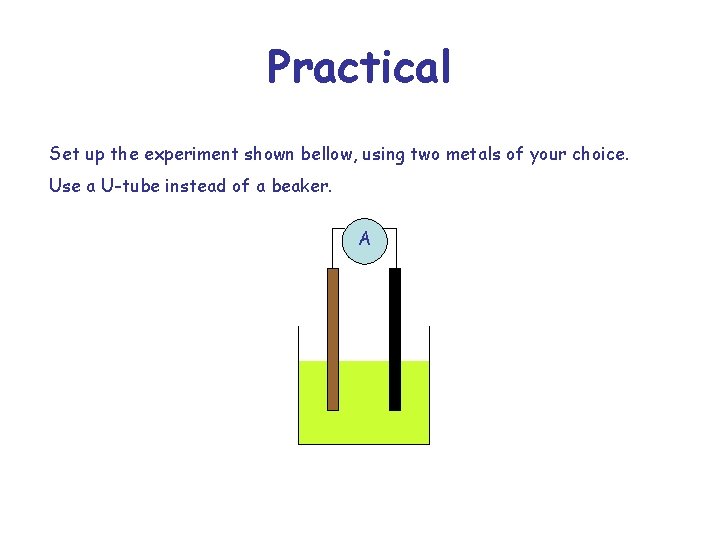
Practical Set up the experiment shown bellow, using two metals of your choice. Use a U-tube instead of a beaker. A

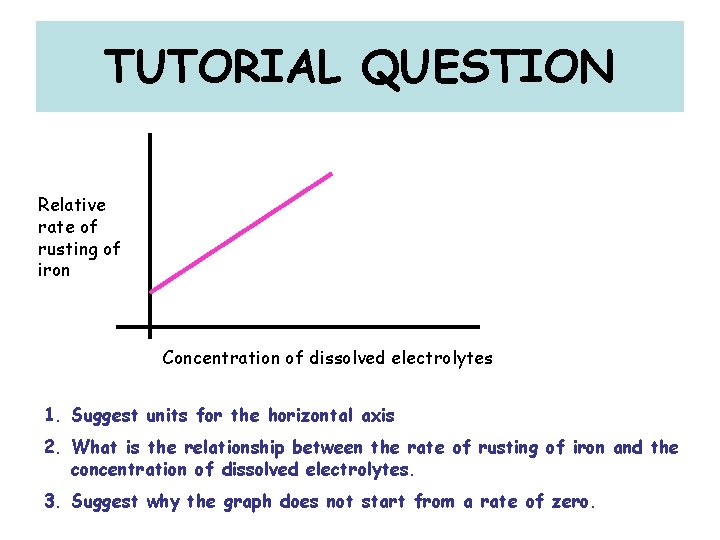
TUTORIAL QUESTION Relative rate of rusting of iron Concentration of dissolved electrolytes 1. Suggest units for the horizontal axis 2. What is the relationship between the rate of rusting of iron and the concentration of dissolved electrolytes. 3. Suggest why the graph does not start from a rate of zero.

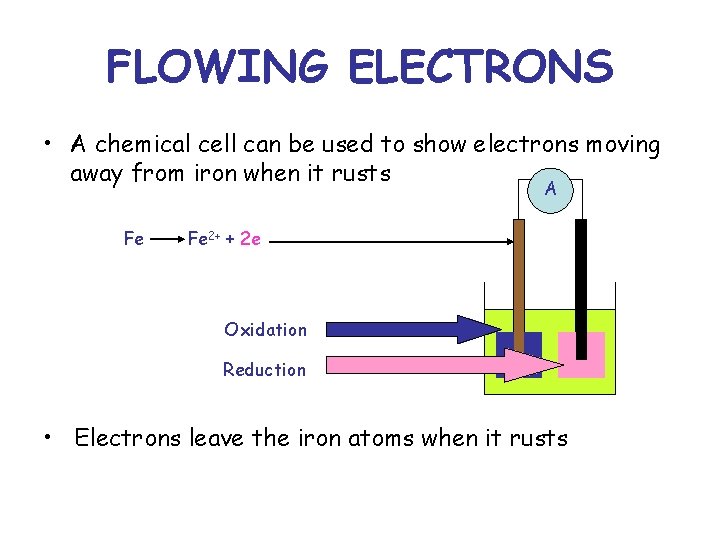
FLOWING ELECTRONS • A chemical cell can be used to show electrons moving away from iron when it rusts A Fe Fe 2+ + 2 e Oxidation Reduction • Electrons leave the iron atoms when it rusts

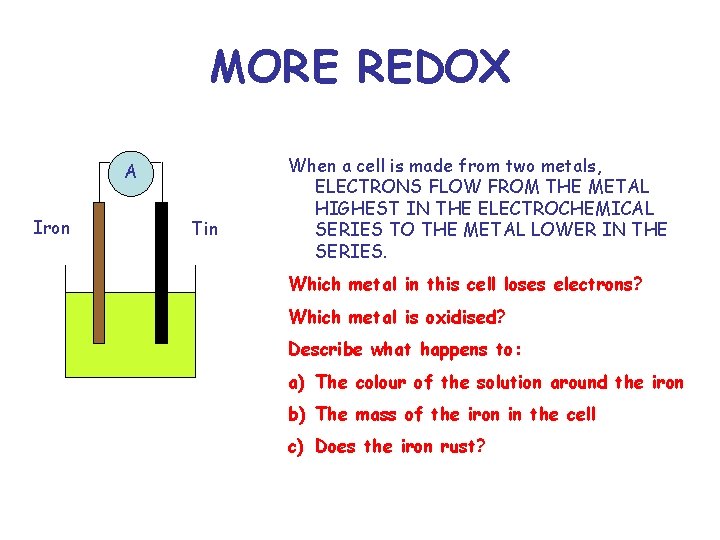
MORE REDOX A Iron Tin When a cell is made from two metals, ELECTRONS FLOW FROM THE METAL HIGHEST IN THE ELECTROCHEMICAL SERIES TO THE METAL LOWER IN THE SERIES. Which metal in this cell loses electrons? Which metal is oxidised? Describe what happens to: a) The colour of the solution around the iron b) The mass of the iron in the cell c) Does the iron rust?

MORE REDOX 2 A Iron When a cell is made from two metals, ELECTRONS FLOW FROM THE METAL HIGHEST IN THE ELECTROCHEMICAL Magnesium SERIES TO THE METAL LOWER IN THE SERIES. Which metal in this cell loses electrons? Which metal is oxidised? Describe what happens to: a) The colour of the solution around the iron b) The mass of the iron in the cell c) Does the iron rust?

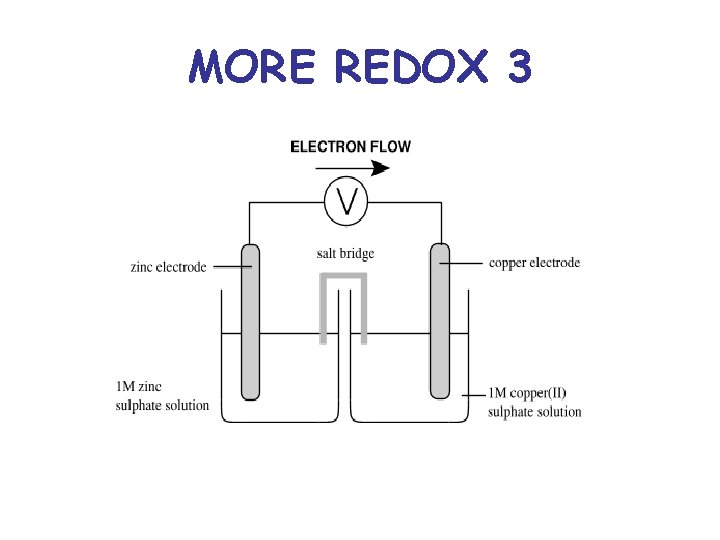
MORE REDOX 3

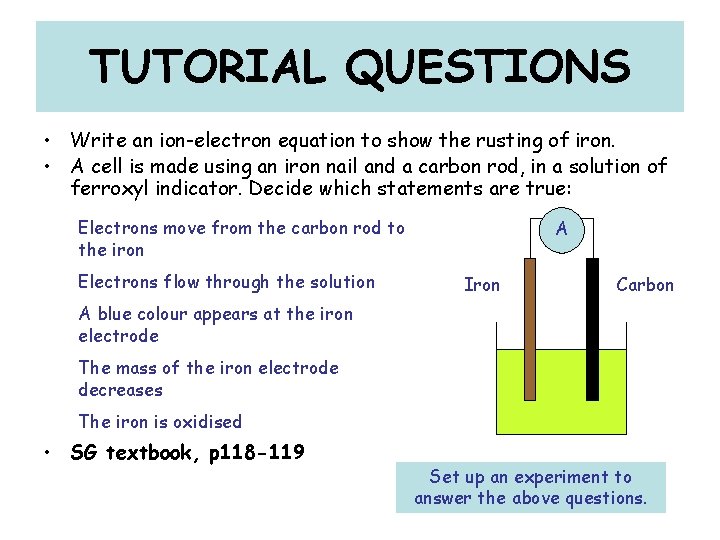
TUTORIAL QUESTIONS • Write an ion-electron equation to show the rusting of iron. • A cell is made using an iron nail and a carbon rod, in a solution of ferroxyl indicator. Decide which statements are true: Electrons move from the carbon rod to the iron Electrons flow through the solution A Iron Carbon A blue colour appears at the iron electrode The mass of the iron electrode decreases The iron is oxidised • SG textbook, p 118 -119 Set up an experiment to answer the above questions.

PREVENTING CORROSION There are two types of way to help prevent corrosion: • Physical protection • Chemical protection PHYSICAL PROTECTION Stops oxygen and water coming into contact with the metal: - Paint - Oil or grease - Plastic coating - Tin plating – covering in molten tin - Galvanising – covering in molten zinc (more expensive) - Cathodic protection - Electroplating Carry out electroplating practical (7. 2)

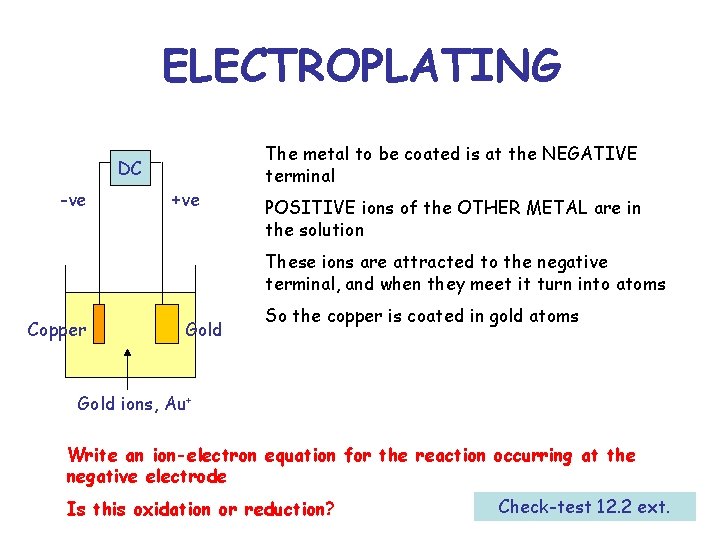
ELECTROPLATING DC -ve +ve The metal to be coated is at the NEGATIVE terminal POSITIVE ions of the OTHER METAL are in the solution These ions are attracted to the negative terminal, and when they meet it turn into atoms Copper Gold So the copper is coated in gold atoms Gold ions, Au+ Write an ion-electron equation for the reaction occurring at the negative electrode Is this oxidation or reduction? Check-test 12. 2 ext.

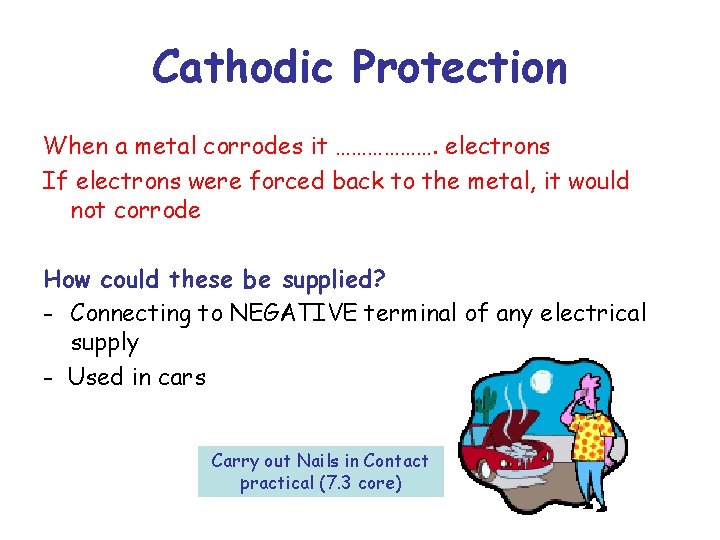
Cathodic Protection When a metal corrodes it ………………. electrons If electrons were forced back to the metal, it would not corrode How could these be supplied? - Connecting to NEGATIVE terminal of any electrical supply - Used in cars Carry out Nails in Contact practical (7. 3 core)

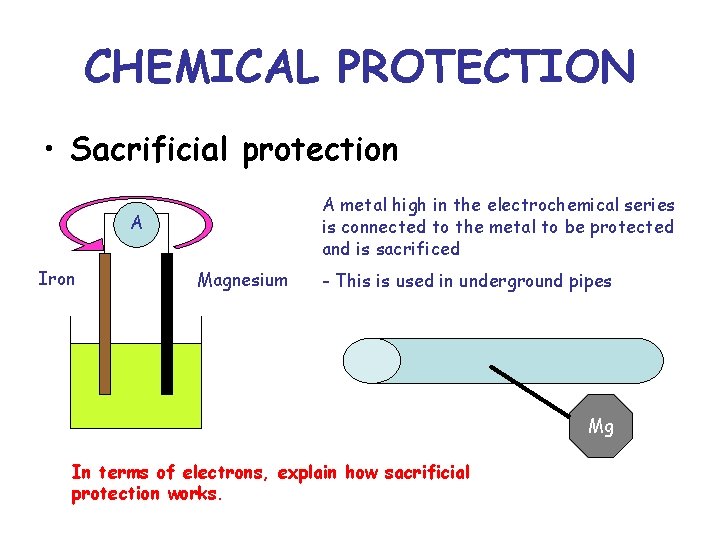
CHEMICAL PROTECTION • Sacrificial protection A metal high in the electrochemical series is connected to the metal to be protected and is sacrificed A Iron Magnesium - This is used in underground pipes Mg In terms of electrons, explain how sacrificial protection works.

• Galvenising

TUTORIAL QUESTIONS 1. a) Why does coating steel with plastic prevent corrosion? b) Chromium-plated steel corrodes quickly if scratched. What does this tell you about the reactivity of chromium? 2. Explain how zinc gives sacrificial protection to steel. 3. a) Why should a copper roof not be held in position with iron nails? b) Suggest a metal which the nails should be made of. Why? 4. Explain what is meant by “electroplating” 5. What name is given to the process where steel is protected by coating it with zinc? 6. Assessment test 12. 1 + 12. 2

PRACTICE EXAM QUESTIONS SG textbook, p 120 -121 Questions for SG textbook, p 43 -44