CORROSION ENVIRONMENTS Corrosive environments include atmosphere aqueous solutions








- Slides: 11

CORROSION ENVIRONMENTS �Corrosive environments include; �atmosphere, �aqueous solutions, �molten salts, �liquid metals. ATMOSPHER E - WATER - SOIL

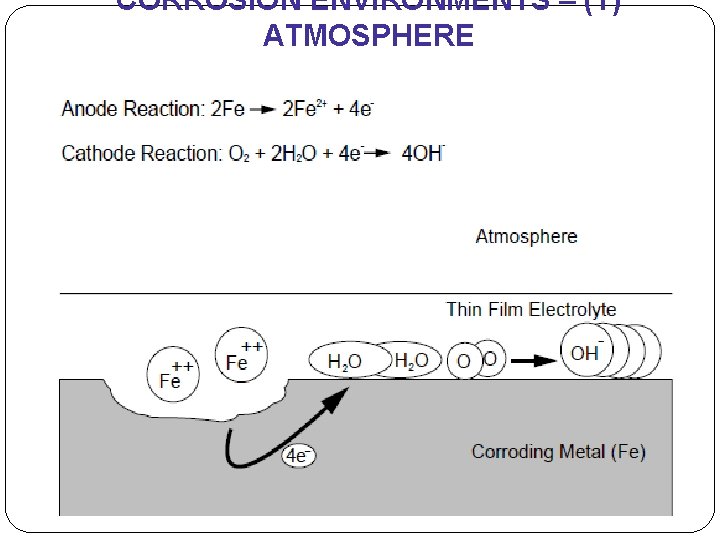
ENVIRONMENT (1) - ATMOSPHERE �Atmospheric corrosion - greatest losses. �the corrosion of materials exposed to air & its pollutants, rather than immersed in a liquid. �Categories – dry, damp, wet �Severity depends on locations – rural, urban, industrial, marine (page 2) �primary agent; - moisture containing dissolved oxygen primary agent! - sulfur compounds - sodium chloride may also contribute.

ENVIRONMENT (1) - ATMOSPHERE (a) Rural; � the least corrosive - does not contain chemical pollutants, but contain organic & inorganic particulates. The principal corrodents are moisture, oxygen & carbon dioxide (CO 2). (b) Urban; � similar to rural- with little industry. � Additional contaminants are of the SOx & NOx from motor vehicles domestic fuel emissions. (c) Industrial; � Has heavy industrial processing facilities that contain concentrations of sulphur dioxide, chlorides, phosphates & nitrates. � Highly corrosive (d) Marine; � Highly corrosive due to fine windswept chloride particles that get deposited on surfaces. � Corrosivity significantly dependent on wind direction, wind speed, & distance from the coast.

CORROSION ENVIRONMENTS – (1) ATMOSPHERE

CORROSION ENVIRONMENTS – (2) WATER � variety of compositions & corrosion characteristics. � Can be classified into fresh water & seawater �(a) Fresh water (surface water & groundwater) normally contains dissolved oxygen ability to dissolve to some degree every substance, either on the earth’s crust & in atmosphere. � Important constituents can be classified into; (1) Dissolved gases; oxygen, nitrogen, carbon dioxide, ammonia, sulphurous gases. (2) Mineral constituents; hardness salts (calcium & magnesium salts content), sodium salts (chloride, sulphate, nitrate, bicarbonate), salts of heavy metals, & silica. (3) Organic matter; animal & vegetable origin, oil, trade waste (including agricultural) constituents, & synthetic detergents. (4) Microbiological forms; various types of algae **slime&=slimelendir

WATER �(b) Seawater contains approximately 3. 5% salt (predominantly sodium chloride) - chloride ions (Na+) being the largest constituent. �Seawater is more corrosive than fresh water. �Seawater systems used by many industries e. g shipping, offshore oil & gas production, power plants, & coastal industrial plants; used for cooling purposes, firefighting, oilfield water injection, & desalination plants. �Frequently producing pitting & crevice corrosion. � Effect of flow velocity (v) �Velocity influences the corrosion behaviour of materials in seawater system. � Effect of temperature (T) �Corrosion of carbon steel increases approximately 50% between the winter (average temperature 7°C) & summer (27°C to 29°C).

CORROSION ENVIRONMENTS – (2) WATER �Important properties of seawater; �High salt concentration �High electrical conductivity �Relatively high & constant p. H �High solubility for gases (O 2 & CO 2) �The presence of myriad (various) of organic compounds �Existence of biological life – microfouling (bacteria, slime), macrofouling (seaweed, mussels, barnacles or many kinds of animals & fish) **fouling = pembusukan **barnacles = teritip

CORROSION ENVIRONMENTS – (3) SOIL �Soils - has wide range of compositions & susceptibilities to corrosion. �Soil is an aggregate of; - minerals, - organic matter, - water, - moisture, - various forms of bacteria, - gases (mostly air) It is formed by the combined weathering action of wind & water, & also organic decay.

CORROSION ENVIRONMENTS – (3) SOIL �Cast iron & plain carbon steels are found most economical for underground structures. �Aging of buried infrastructure; Øpipelines of oil, gas & water Øburied storage tanks; Øelectrical communication cables & conduits; Øanchoring systems; Øcasings of well & shaft.

CORROSION ENVIRONMENTS – (3) SOIL �Microbiologically influenced corrosion (MIC) - corrosion influenced by the presence & activities of microorganisms and/or their metabolites (the product produced through their metabolism). �Example – bacteria, fungi & other microorganisms. �Rapid corrosion failures have been observed in soil as a result of microbial action. �Types of microorganisms associated with corrosion damage in soils are; �Anaerobic bacteria: produce highly corrosive species as part of their metabolism. �Aerobic bacteria: produce corrosive mineral acids. �Fungi: produce corrosive by-products in their metabolism, such as organic acids. Apart from metals & alloys, they can degrade organic coatings & wood.

~ end ~