Correct Site Correct Patient Correct Procedure Documentation Audit






- Slides: 11

Correct Site Correct Patient Correct Procedure Documentation Audit Team Membership Paula Hindle, RN, MSN Vice President Chief Nurse Executive Mary Altier RN, MSN Nursing Quality Specialist Peggy Vorrier RN, MS Administrative Director Surgical Services Gigi Marinakos-Trulis Data Analyst Jo Quetsch RN MA-OM Manager Operating Room Jeri Katsaros RN, BSN Manager Same Day Surgery/PAR

Department Membership • Main Operating Room • Labor and Delivery • Newborn Nursery • BICU, NICU, PICU • 4 SICU, 2 WICU, 2 CCU • Special Procedures • Cardiographics • EP Lab • Cardiac Cath Lab • Pulmonary Function Lab • Nuclear Medicine • • • Ultrasound GI Lab Breast Imaging Dermatology Pain Clinic ENT Clinic Oak Brook Terrace 1 LOC Surgery Center Ambulatory Surgery Center • Cancer Center • Oral Health Center

Opportunity Statement There is an opportunity at LUHS to assure compliance with the 2005 National Patient Safety Goal of the Universal Protocol for preventing wrong site, wrong procedure and wrong patient surgery. Project goal: Improve the submission and documentation rates for compliance with proper consents, site verification and time-out procedures.

Most Likely Causes Identified • Knowledge deficit regarding use of tool • Knowledge deficit regarding proper submission of form • Lack of understanding of – Universal Protocol – Which surgeries/procedures are included in protocol – Which surgeries/procedures require site marking – Number of personnel required for a “timeout”

Solutions Implemented • Education sessions for key stakeholders regarding use of tool • Random sampling of verification checklists performed quarterly: 4 th quarter 2003 -4 th quarter 2005 • Quarterly reports to clinical departments

Data Analysis • 9 data collection periods sinception • 26 -29 departments submitted forms for audits • Total sheets audited N=13, 696 • Data elements measured: – Consents signed, site marked, time-out completed.

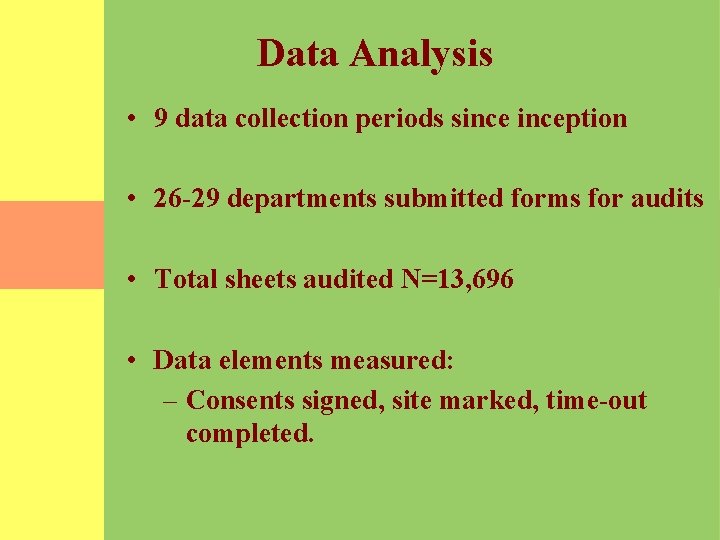
Form Submissions The number of departments submitting forms for audit has increased by 77% since project inception.

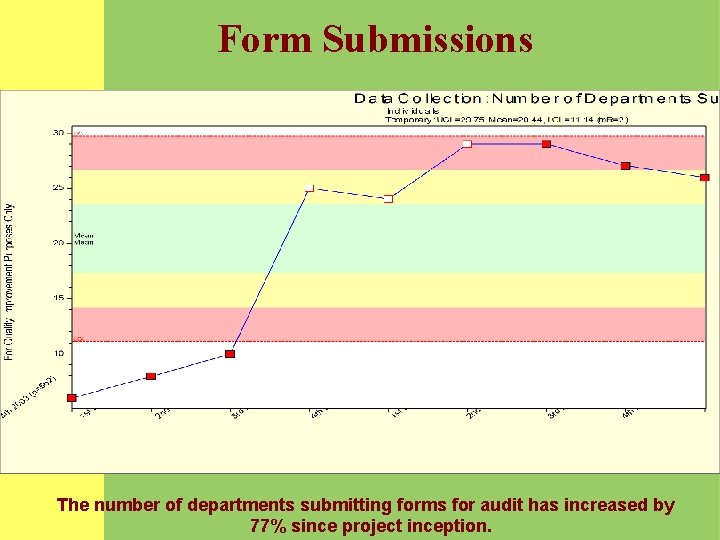
Consents Properly Documented Definition: Percent of appropriate documentation demonstrating the department is in compliance with the Operative and Invasive Procedure Verification policy. (#13. 0015. 6) Data Source: Original data extracted from LUHS Site Verification Sheets by RN and Data Analyst. Analysis: LUHS performance is stable with a mean of 95%.

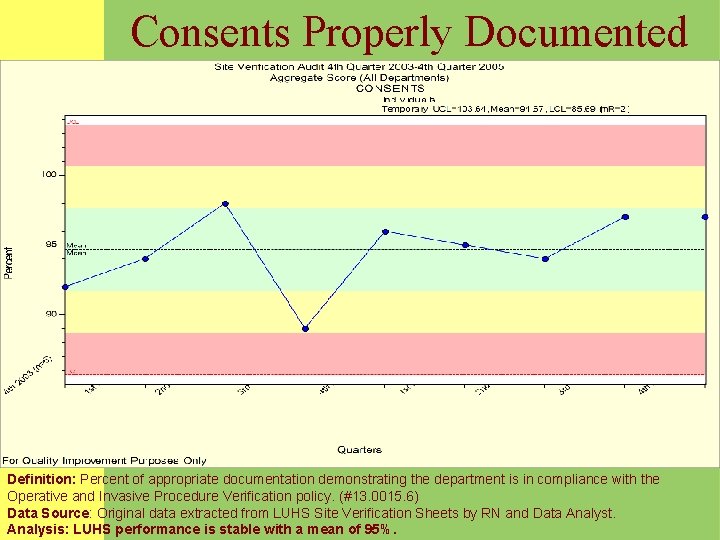
Site Verification Properly Documented Definition: Percent of appropriate documentation demonstrating the department is in compliance with the Operative and Invasive Procedure Verification policy. (#13. 0015. 6) Data Source: Original data extracted from LUHS Site Verification Sheets by RN and Data Analyst. Analysis: LUHS mean performance is at 81%. Recent month data demonstrates improvement.

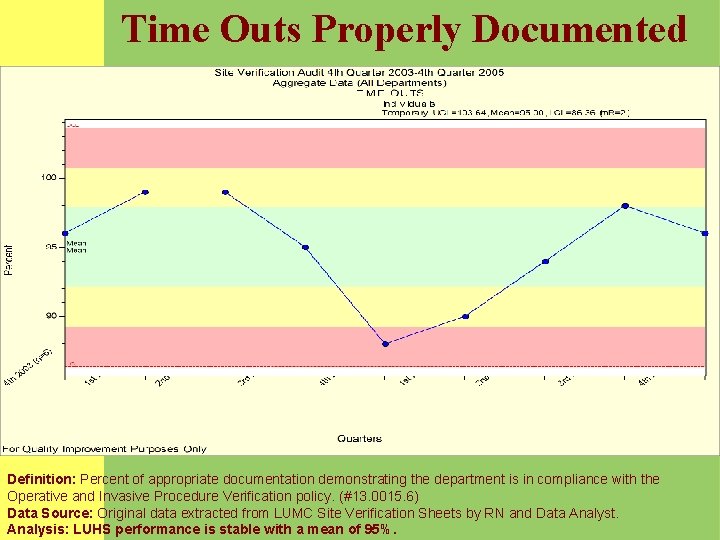
Time Outs Properly Documented Definition: Percent of appropriate documentation demonstrating the department is in compliance with the Operative and Invasive Procedure Verification policy. (#13. 0015. 6) Data Source: Original data extracted from LUMC Site Verification Sheets by RN and Data Analyst. Analysis: LUHS performance is stable with a mean of 95%.

Next Steps • Revision of form: 1 st Quarter 2006 with implementation targeted for 3 rd quarter 2006 collection period • Revision of Operative procedure policy: Quarter 2 2006 • In-service to all key stakeholders: both inpatient and Ambulatory sites regarding revised form/policy • Monitor departments for compliance • Quarterly reports to clinical departments provide feedback on performance