COREVALVE RCT 3 YR OUTCOMES JOSEPHINE MAK WAIKATO
























- Slides: 32

COREVALVE RCT 3 YR OUTCOMES JOSEPHINE MAK WAIKATO CARDIOTHORACIC UNIT JOURNAL CLUB

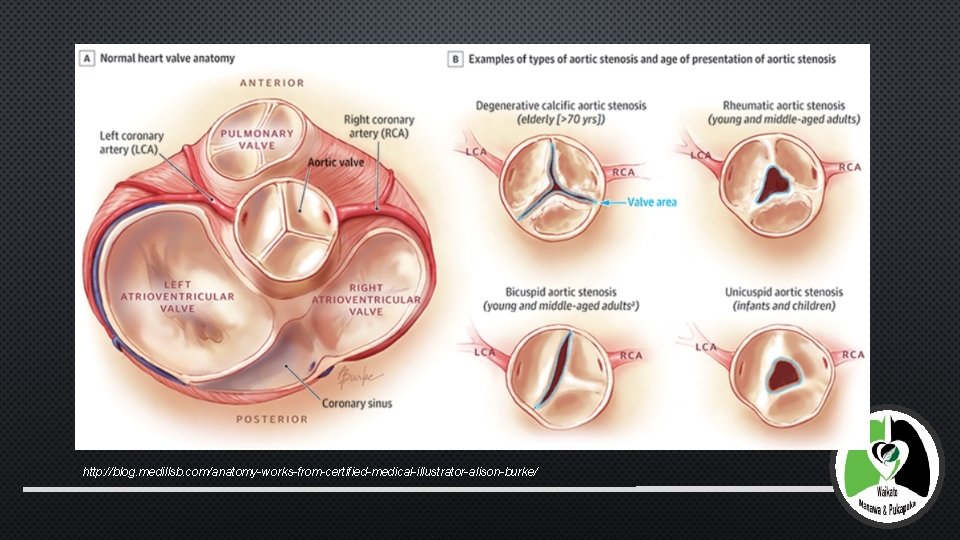
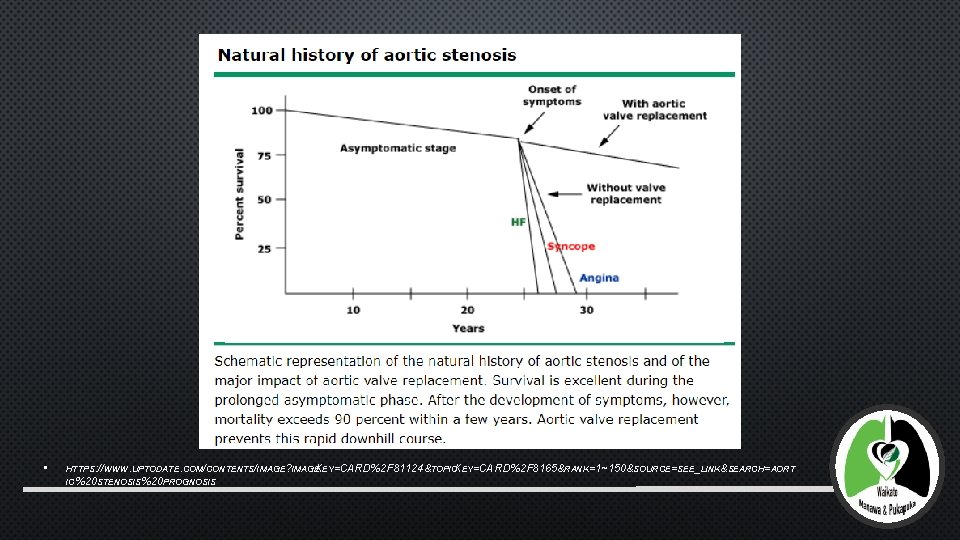
AORTIC STENOSIS • AORTIC STENOSIS IS A DEBILITATING DISEASE IN ELDERLY PEOPLE • CAUSES LVOT OBSTRUCTION • CLASSICAL MANIFESTATIONS - HEART FAILURE, SYNCOPE, ANGINA • POOR PROGNOSIS AFTER SYMPTOM ONSET – ON MEDICAL TREATMENT, MORTALITY FROM THE ONSET OF SYMPTOMS IS APPROXIMATELY 25% AT 1 YEAR AND 50% AT 2 YEARS

http: //blog. medillsb. com/anatomy-works-from-certified-medical-illustrator-alison-burke/

• HTTPS: //WWW. UPTODATE. COM/CONTENTS/IMAGE? IMAGE KEY=CARD %2 F F 81124&TOPICKEY=CARD %2 F F 8165&RANK=1~150&SOURCE=SEE_LINK&SEARCH=AORT =CARD%2 IC%20 STENOSIS%20 PROGNOSIS

SURGICAL AVR VS TAVI • CURRENT STANDARD OF TREATMENT IS SURGICALAVR FOR SEVEREAS • OFTEN PATIENTS UNSUITABLE FOR SURGICALAVR – HIGH RISK OF DEATH • TRANSCATHETER AORTIC VALVE IMPLANTATION / REPLACEMENT TAVR) ( • VALVE LOADED IN A SPECIALISED DELIVERY CATHETER, ADVANCED VIA WIRE EITHER THROUGH FEMORAL ARTERY (RETROGRADE) OR CHEST INCISION (TRANSAPICAL) • CURRENTLY A NUMBER OF TRIALS BEGINNING TO COMPARE OUTCOMES INSAVR VS TAVI – COREVALVE, PARTNER STUDY

http: //www. nature. com/nrcardio/journal/v 13/n 6/fig_tab/nrcardio. 2016. 43_F 1. html http: //www. medtronic. com/us-en/about/news/media-resources/media-kits/medtronic-corevalve-system. html

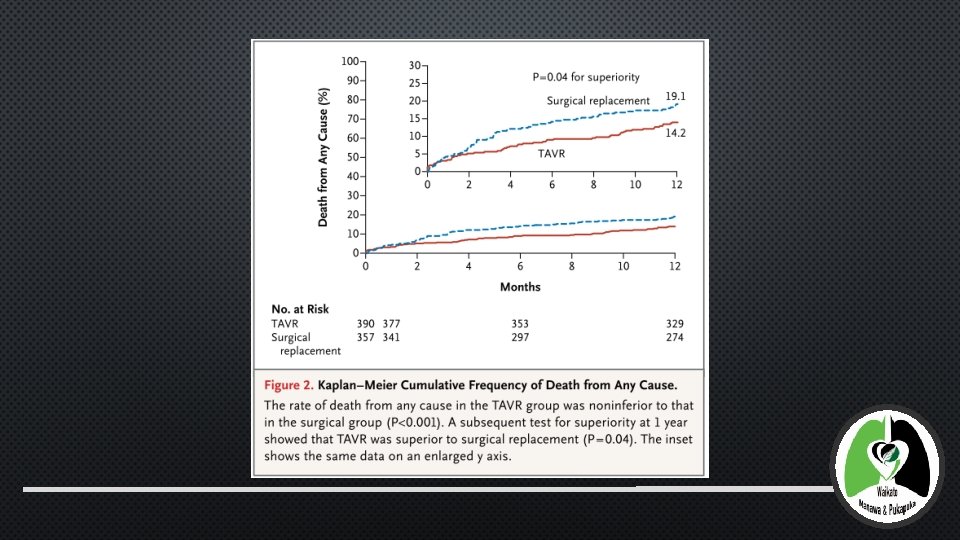
1 YR OUTCOMES • 795 PATIENTS UNDERWENT RANDOMIZATION AT 45 CENTRES INUTHE NITED STATES. • POWERED TO LOOK FOR NON INFERIORITY INTAVR GROUP AS COMPARED TO SAVR • NONINFERIORITY MARGIN OF 7. 5%, ASSUMING A 1: 1 RATIO IN THE TREATMENT ASSIGNMENTS AND RATE OF DEATH AT 1 YEAR OF 20% IN EACH GROUP - 355 PATIENTS REQUIRED IN EACH GROUP FOR POWER OF 80% AT A ONE-SIDED ALPHA LEVEL OF 0. 05. ENROL 790 PATIENTS TO ACCOUNT FOR 10% LOSS TO FOLLOW UP • IN AS-TREATED ANALYSIS, RATE OF DEATH FROM ANY CAUSE AT 1 YEAR WAS SIGNIFICANTLY LOWER IN THE TAVR THAN SAVR (14. 2% VS. 19. 1%) • ABSOLUTE REDUCTION IN RISK OF 4. 9 PERCENTAGE POINTS (UPPER BOUNDARY OF THE 95% CONFIDENCE INTERVAL, − 0. 4; P<0. 001 FOR NONINFERIORITY P =; 0. 04 FOR SUPERIORITY). • TAVR NON-INFERIOR WITH RESPECT TO ECHO INDEXES OF VALVE STENOSIS, FUNCTIONAL STATUS, AND QUALITY OF LIFE. • SUGGESTION OF A REDUCTION IN THE RATE OF MAJOR ADVERSE CARDIOVASCULAR AND CEREBROVASCULAR EVENTS AND NO INCREASE IN THE RISK OF STROKE


HYPOTHESIS • “THERE IS SUSTAINED 3 -YEAR CLINICAL BENEFIT OF SELF-EXPANDINGTAVR OVERSAVR IN PATIENTS WITH AORTIC STENOSIS AT INCREASED RISK FOR SURGERY” PRIMARY OUTCOME • ALL CAUSE MORTALITY

HYPOTHESIS SECONDARY OUTCOMES • • ALL STROKE • • ALL-CAUSE MORTALITY OR MAJOR STROKE • • LIFE-THREATENING OR DISABLING BLEEDING • • VALVE ENDOCARDITIS MAJOR STROKE PACEMAKER IMPLANTATION, VALVE THROMBOSIS MAJOR ADVERSE CARDIOVASCULAR AND CEREBROVASCULAR EVENTS MACCE ( ACCE) (DEATH, MYOCARDIAL M INFARCTION, STROKE, OR REINTERVENTION). • NYHA FUNCTIONAL CLASS • ECHO FINDINGS ESTIMATIONS OF AVA, MEAN AORTIC VALVE GRADIENT, AND AORTIC REGURGITATION WERE USED FOR THIS ANALYSIS.

METHODOLOGY

METHODS – ENTRY CRITERIA • • MULTI-CENTRE, PROSPECTIVE, RANDOMISED, NON-INFERIORITY TRIAL 45 SITES INUSA, FEBRUARY 2011 THROUGHSEPTEMBER 2012 995 PATIENTS WERE SCREENED ELIGIBLE FOR RANDOMISATION • SEVERE AS AND NYHA CLASSII OR HIGHERAVA ( OF < 0. 8 OR ANAV INDEX OF <0. 5 CM 2/M 2 PER SQUARE METER EITHER A MEANAV GRADIENT OF MORE THAN 40 MM HG OR A PEAK AORTIC-JET VELOCITY OF <4. 0 M/S) • INCREASED RISK FOR UNDERGOING SAVR – AS JUDGED BY TWO CARDIAC SURGEONS AND ONE INTERVENTIONAL CARDIOLOGIST AT THE INVESTIGATIVE SITE - RISK OF DEATH WITHIN 30 DAYS AFTER SURGERY >15% AND IRREVERSIBLE COMPLICATIONS < 50% USING STS SCORING SYSTEM • INDEPENDENT CLINICAL EVENTS COMMITTEE ADJUDICATED ALL MAJOR CLINICAL EVENTS • INDEPENDENT DATA SAFETY MONITORING BOARD PROVIDED STUDY OVERSIGHT • INSTITUTIONAL REVIEW BOARD AT EACH SITE

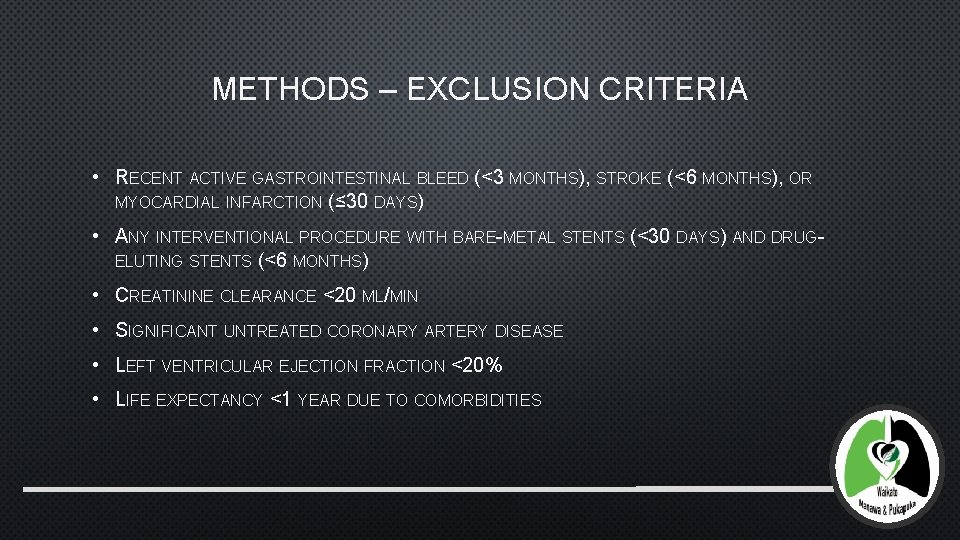
METHODS – EXCLUSION CRITERIA • RECENT ACTIVE GASTROINTESTINAL BLEED (<3 MONTHS), STROKE (<6 MONTHS), OR MYOCARDIAL INFARCTION (≤ 30 DAYS) • ANY INTERVENTIONAL PROCEDURE WITH BARE-METAL STENTS (<30 DAYS) AND DRUGELUTING STENTS (<6 MONTHS) • CREATININE CLEARANCE <20 ML/MIN • SIGNIFICANT UNTREATED CORONARY ARTERY DISEASE • LEFT VENTRICULAR EJECTION FRACTION <20% • LIFE EXPECTANCY <1 YEAR DUE TO COMORBIDITIES

METHODS – STUDY PROTOCOL • RANDOMLY ASSIGNED TO TAVR ORSAVR IN 1: 1 MANNER • STRATIFIED BY STUDY SITE AND INTENDED ACCESS SITE (ILIOFEMORAL OR NON ILIOFEMORAL) TO ENSURE PROPORTIONAL ASSIGNMENT • TAVR: • BIO-PROSTHETIC VALVE SIZE CHOSEN ACCORDING TO PERIMETER BASED DIAMETER OF THE SCREENING MULTIDETECTOR CT • DAPT (ASPIRIN AND CLOPIDOGREL) RECOMMENDED BEFORE AND FOR 3 MONTHS POSTTAVR • SAVR: • SITE-SPECIFIC STANDARD SURGICAL TECHNIQUES • VALVE TYPE & SIZE SELECTED BY INDIVIDUAL SURGEON BY DIRECT ANATOMIC MEASUREMENT • ASPIRIN PRESCRIBED INDEFINITELY IN ALL PATIENTS (“INCLUDING THOSE ON WARFARIN”)

METHODS – ENDPOINTS • AN INDEPENDENT CLINICAL EVENTS COMMITTEE EVALUATED 3 -YEAR CLINICAL EVENTS USING THE VALVE ACADEMIC RESEARCH CONSORTIUM-1 CRITERIA • PRIMARY END POINT WAS ALSO ANALYSED IN THE INTENTION-TO-TREAT POPULATION, WHICH INCLUDED ALL THE PATIENTS WHO HAD UNDERGONE RANDOMIZATION • CLINICAL SITES REPORTED NYHA CLASS • CLINICAL SITES REPORTED ECHO FINDINGS -WORSENING AORTIC VALVE GRADIENTS WERE DEFINED AS A >50% INCREASE IN THE AORTIC VALVE GRADIENT FROM 1 -MONTH TO 3 -YEAR FOLLOW-UP

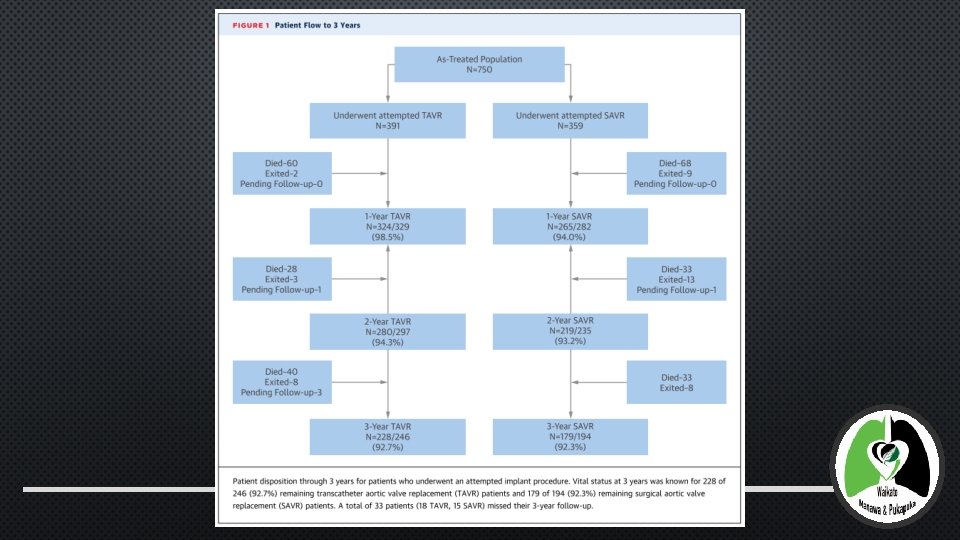
METHODS – STATISTICAL ANALYSIS • STATISTICAL ANALYSIS EVALUATED THE AS-TREATED POPULATION • IE ALL PATIENTS WHO UNDERWENT AN ATTEMPTED IMPLANTATION • CATEGORICAL VARIABLES WERE COMPARED USING THEFISHER’S EXACT TEST OR CHI-SQUARE TEST. CONTINUOUS VARIABLES ARE PRESENTED AS THE MEAN +/SD, COMPARED USING THE STUDENT T TEST • KAPLAN-MEIER ESTIMATES WERE USED TO CONSTRUCT THE SURVIVAL CURVES ON THE BASIS OF ALL AVAILABLE FOLLOW-UP DATA FOR THE TIME-TO-EVENT ANALYSIS • DIFFERENCES IN EVENTS RATES BETWEEN THETAVR AND SAVR GROUPS WERE EVALUATED USING THE LOG-RANK TEST • ECHO MEASUREMENTS WERE EVALUATED USING A 2 -SAMPLESTUDENT T TEST OR THE WILCOXON RANK SUM TEST FOR CONTINUOUS VARIABLES AND THEMANTEL-HAENSZEL TEST FOR CATEGORICAL VARIABLES • ALL TESTING USED A 2 -SIDED ALPHA LEVEL OF 0. 05

RESULTS


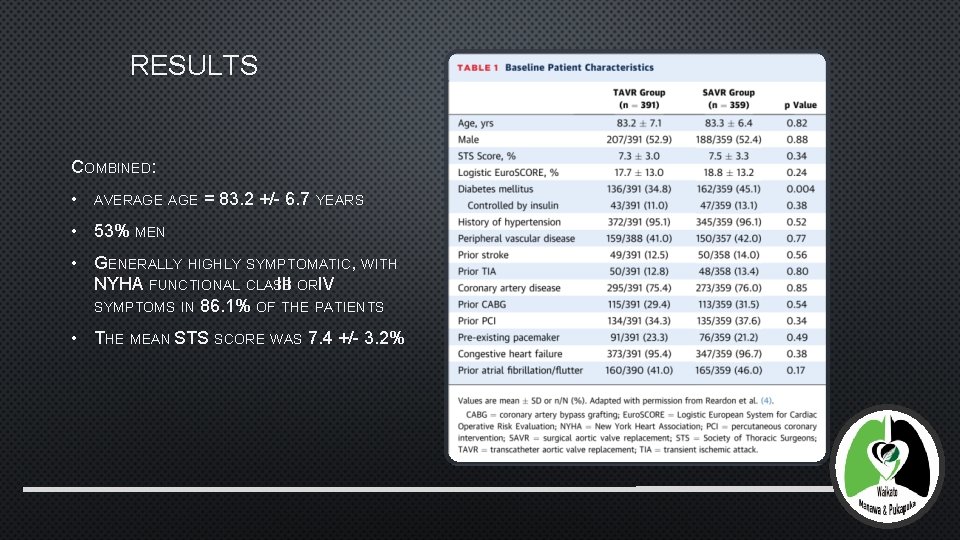
RESULTS COMBINED: • AVERAGE = 83. 2 +/- 6. 7 YEARS • 53% MEN • GENERALLY HIGHLY SYMPTOMATIC, WITH NYHA FUNCTIONAL CLASS III ORIV SYMPTOMS IN 86. 1% OF THE PATIENTS • THE MEAN STS SCORE WAS 7. 4 +/- 3. 2%

RESULTS

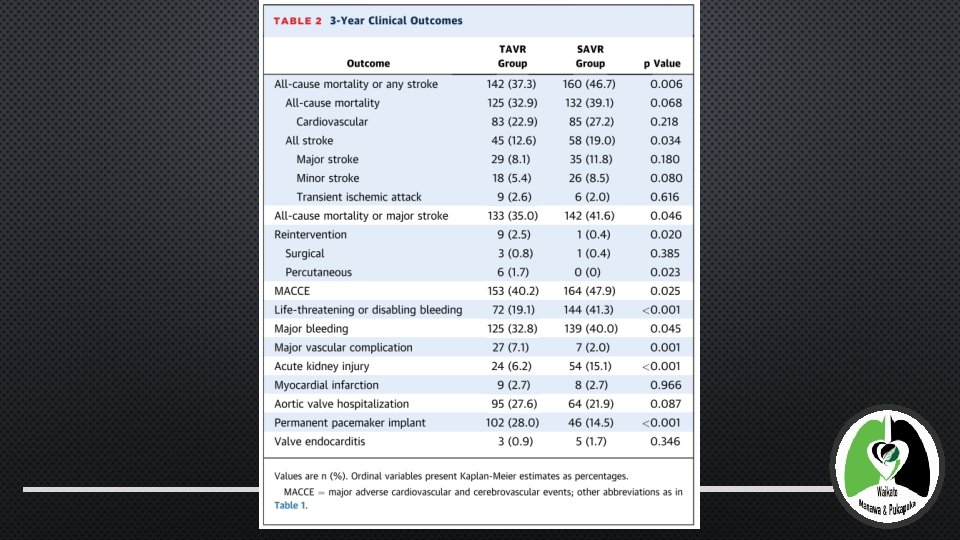
RESULTS (TABLES AND DIAGRAMS) • TAVR: • 20. 1% RELATIVE REDUCTION IN THE OCCURRENCE OF ALL-CAUSE MORTALITY OR STROKE AT 3 YEARS IN TAVR COMPARED WITHSAVR (P <0. 006) (WITH AN ABSOLUTE RISK REDUCTION OF 9. 4%) • NONSIGNIFICANT 15. 9% RELATIVE RISK REDUCTION IN ALL-CAUSE MORTALITY • 33. 7% RELATIVE RISK REDUCTION IN ALL STROKE • 15. 9% RELATIVE RISK REDUCTION IN ALL-CAUSE MORTALITY OR MAJOR STROKE • 16. 1% RELATIVE RISK REDUCTIONMACCE IN

RESULTS • LIFE-THREATENING OR MAJOR BLEEDING AND AKI MORE COMMON IN THESAVR GROUP • VASCULAR COMPLICATIONS, REINTERVENTION, AND THE NEED FOR NEW PERMANENT PACEMAKERS WERE MORE COMMON IN THETAVR (MOST WITHIN FIRST 30 DAYS) • 3 YR AV GRADIENTS WERE LOWER TAVI IN THAN SAVR (MEAN AORTIC VALVE GRADIENT 7. 62 +/- 3. 57 MM HG VS. 11. 40 +/- 6. 81 H MM G IN SURGICAL PATIENTS; P < 0. 001) • MODERATE OR SEVERE AR WAS HIGHER INTAVR P < 0. 001) • NO EVIDENCE OF CLINICAL VALVE THROMBOSIS OR STRUCTURAL VALVE DETERIORATION IN EITHER GROUP • RATES OF WORSENING AORTIC VALVE GRADIENTS, DEFINED AS A >50% INCREASE FROM 1 MONTH TO 3 YEARS, WERE SIMILAR (9. 5% TAVR IN , 12. 6% IN THE SAVR GROUP; P = 0. 38) (6. 8%VS. 0. 0% IN SURGICAL PATIENTS;

RESULTS (TABLES AND DIAGRAMS)

AUTHOR’S CONCLUSION • THEIR FINDINGS SUPPORT USE OFSAVR AS TREATMENT OF CHOICE IN PATIENTS SUBOPTIMAL FOR SURGERY -SUSTAINED 3 YR CLINICAL BENEFIT INSELF-EXPANDING TAVR OVERSAVR • REDUCTIONS IN ALL-CAUSE MORTALITY AND STROKE • IMPROVED VALVE HAEMODYNAMICS (LOWER 3 -YEAR MEAN AORTIC VALVE GRADIENTS AND LARGER EFFECTIVE ORIFICE AREAS ALTHOUGH MORE AR) • NO DIFFERENCE BETWEEN TAVR AND SAVR IN STRUCTURAL VALVE DETERIORATION OVER TIME • “ADDITIONAL STUDIES ARE NEEDED TO VALIDATE OUTCOMES OFTAVR OVER EVEN LONGER FOLLOW-UP INTERVALS AND IN LOWER-RISK PATIENTS WITH SEVERE AORTIC STENOSIS”

DISCUSSION

STRENGTHS OF THE STUDY • FOLLOW UP – STATUS OF 92%-93% OF PATIENTS IN BOTH GROUPS KNOWN • STRATIFICATION OF RANDOMISATION

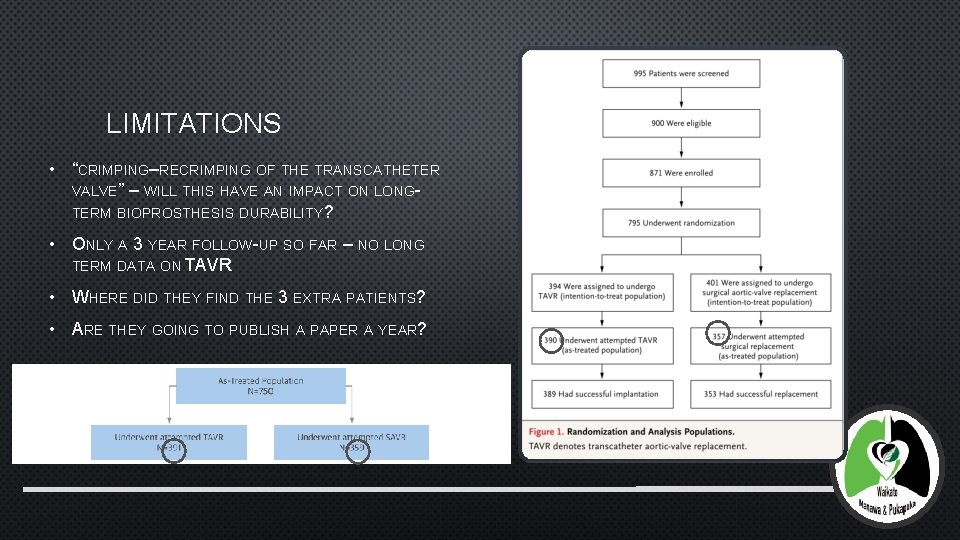
LIMITATIONS • “CRIMPING–RECRIMPING OF THE TRANSCATHETER VALVE” – WILL THIS HAVE AN IMPACT ON LONGTERM BIOPROSTHESIS DURABILITY? • ONLY A 3 YEAR FOLLOW-UP SO FAR – NO LONG TERM DATA ON TAVR • WHERE DID THEY FIND THE 3 EXTRA PATIENTS? • ARE THEY GOING TO PUBLISH A PAPER A YEAR?

LIMITATIONS

LIMITATIONS • MEDTRONIC… • FUNDED THE STUDY • DEVELOPED THE PROTOCOL – IN COLLABORATION WITH A STUDY STEERING COMMITTEE • SELECTED THE 45 CLINICAL SITES • MONITORED THE DATA • MANAGED ALL SOURCE DATA • MANAGED STATISTICAL ANALYSES • STATISTICAL ANALYSES “VALIDATED BYHARVARD CLINICAL RESEARCH INSTITUTE, WITH ALL MAJOR CLINICAL EVENTS REVIEWED BY INDEPENDENT BOARD, AND INDEPENDENT DATA AND SAFETY MONITORING RESPONSIBLE FOR STUDY OVERSIGHT”

IMPACT OF THE STUDY

HOW HAS THE STUDY IMPACTED ON PRACTICE • FURTHER TRIALS ON TAVR ONGOING • EXPANSION OF THEIR USE IN LOWER RISK PATIENTS • SURGICAL VALVES - ? SUTURELESS VALVES • THOSE WITH MORE EXPERIENCE – HOW HAVE YOU PICKED THE VALVE YOU USE AND WHY?

REFERENCES • JOURNAL CLUB PAPER – 3 YR OUTCOMES • HTTP: //WWW. ONLINEJACC. ORG/CONTENT/67/22/2565 • ORIGINAL PAPER • HTTP: //WWW. NEJM. ORG/DOI/10. 1056/ NEJMOA 1400590 • HTTPS: //WWW. UPTODATE. COM/CONTENTS/NATURAL-HISTORY-EPIDEMIOLOGY-ANDPROGNOSIS-OF-AORTIC-STENOSIS • HTTPS: //WWW. UPTODATE. COM/CONTENTS/CLINICAL-MANIFESTATIONS-AND-DIAGNOSIS-OFAORTIC-STENOSIS-IN-ADULTS
 Atu mak
Atu mak Josephine mak
Josephine mak Corevalve vs sapien
Corevalve vs sapien Waikato kindergarten association
Waikato kindergarten association Landfowl
Landfowl Waikato dhb ransomware
Waikato dhb ransomware Waikato stormwater management guideline
Waikato stormwater management guideline Bias rct
Bias rct Rct brisbane
Rct brisbane Rct
Rct 16 décembre 2010
16 décembre 2010 Rct-822
Rct-822 Orthograde vs retrograde root filling
Orthograde vs retrograde root filling Rct children's services
Rct children's services Rct-274
Rct-274 Attrition bias
Attrition bias Bias rct
Bias rct Dr josephine perry
Dr josephine perry Josephine bobeck
Josephine bobeck Alice porter murray
Alice porter murray Fr. vicente balaguer wikipedia
Fr. vicente balaguer wikipedia Who is this?
Who is this? Tone in the story of an hour
Tone in the story of an hour Josephine savarese
Josephine savarese Chinese knotting tutorial
Chinese knotting tutorial Josephine ensign
Josephine ensign Josephine pravinkumar
Josephine pravinkumar Kiyan banuri
Kiyan banuri Instanz aufbauorganisation
Instanz aufbauorganisation Mak ma system korzeniowy palowy
Mak ma system korzeniowy palowy Rudolf mak
Rudolf mak Jozef mak postavy
Jozef mak postavy Mak rastlina
Mak rastlina