Core Valve Evolut R Evolut PRO Core Valve



- Slides: 24

Core. Valve™ Evolut™ R Evolut™ PRO+ Core. Valve Evolut R program: Next Generation Improvement and Design features Pieter Kappetein, CMO, VP Medical Affairs Medtronic

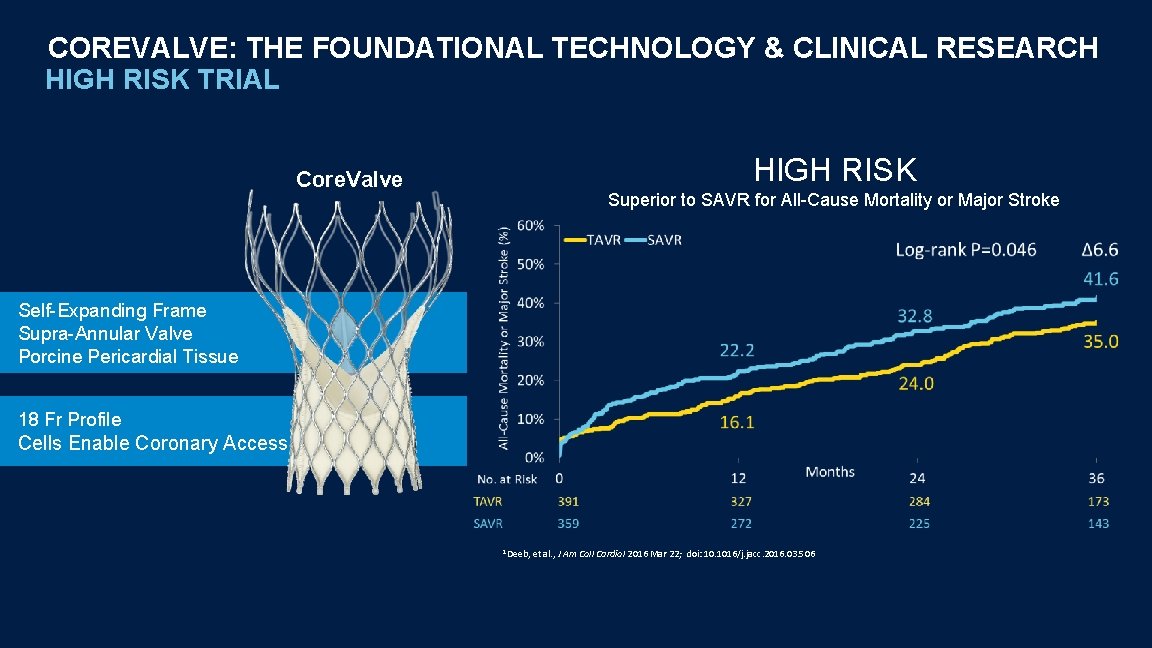
COREVALVE: THE FOUNDATIONAL TECHNOLOGY & CLINICAL RESEARCH HIGH RISK TRIAL HIGH RISK Core. Valve Superior to SAVR for All-Cause Mortality or Major Stroke Self-Expanding Frame Supra-Annular Valve Porcine Pericardial Tissue 18 Fr Profile Cells Enable Coronary Access 1 Deeb, et al. , J Am Coll Cardiol 2016 Mar 22; doi: 10. 1016/j. jacc. 2016. 03. 506

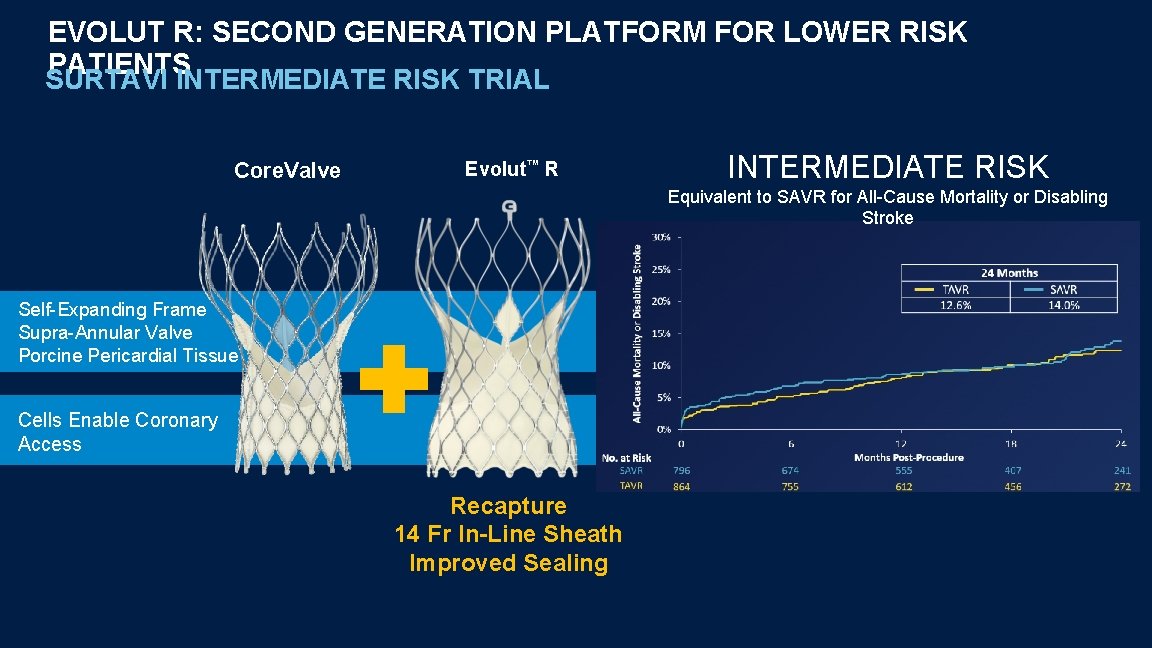
EVOLUT R: SECOND GENERATION PLATFORM FOR LOWER RISK PATIENTS SURTAVI INTERMEDIATE RISK TRIAL Core. Valve Evolut™ R INTERMEDIATE RISK Equivalent to SAVR for All-Cause Mortality or Disabling Stroke Self-Expanding Frame Supra-Annular Valve Porcine Pericardial Tissue Cells Enable Coronary Access Recapture 14 Fr In-Line Sheath Improved Sealing

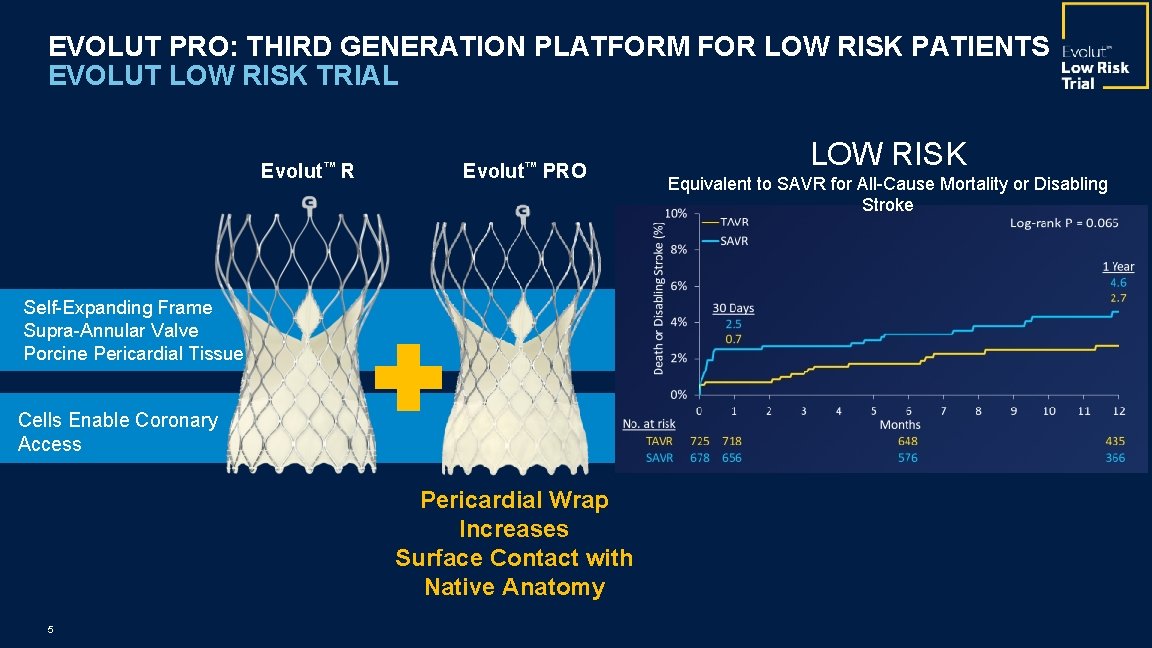
EVOLUT PRO: THIRD GENERATION PLATFORM FOR LOW RISK PATIENTS EVOLUT LOW RISK TRIAL Evolut™ R Evolut™ PRO Self-Expanding Frame Supra-Annular Valve Porcine Pericardial Tissue Cells Enable Coronary Access Pericardial Wrap Increases Surface Contact with Native Anatomy 5 LOW RISK Equivalent to SAVR for All-Cause Mortality or Disabling Stroke

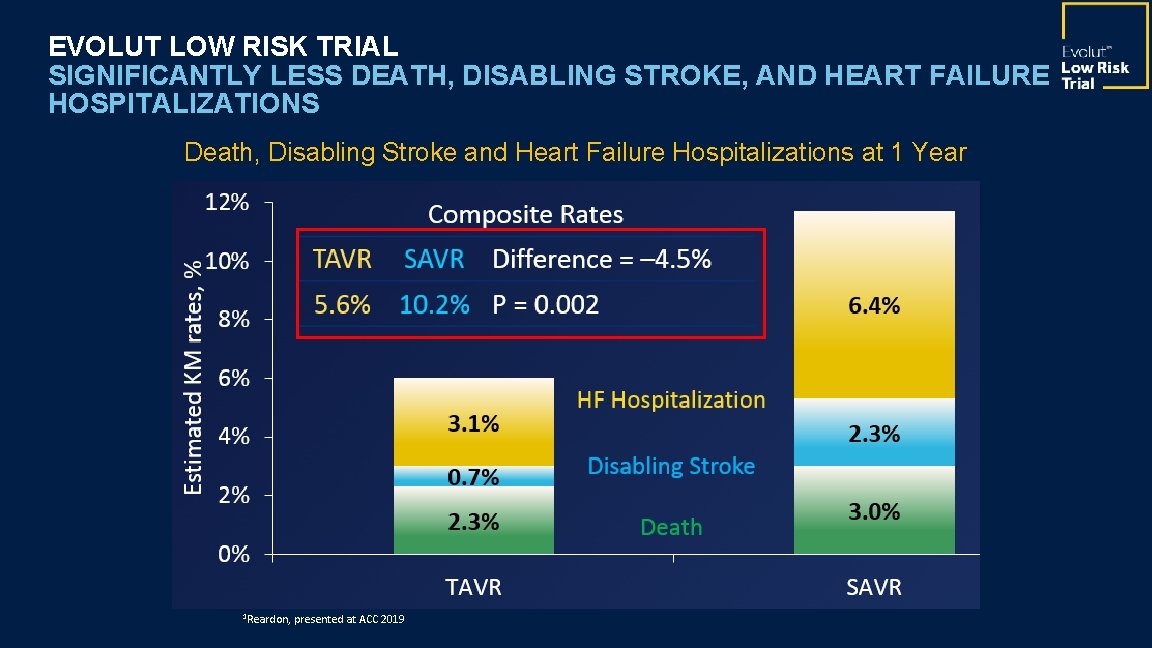
EVOLUT LOW RISK TRIAL SIGNIFICANTLY LESS DEATH, DISABLING STROKE, AND HEART FAILURE HOSPITALIZATIONS Death, Disabling Stroke and Heart Failure Hospitalizations at 1 Year 1 Reardon, presented at ACC 2019

COREVALVE FOUNDATION

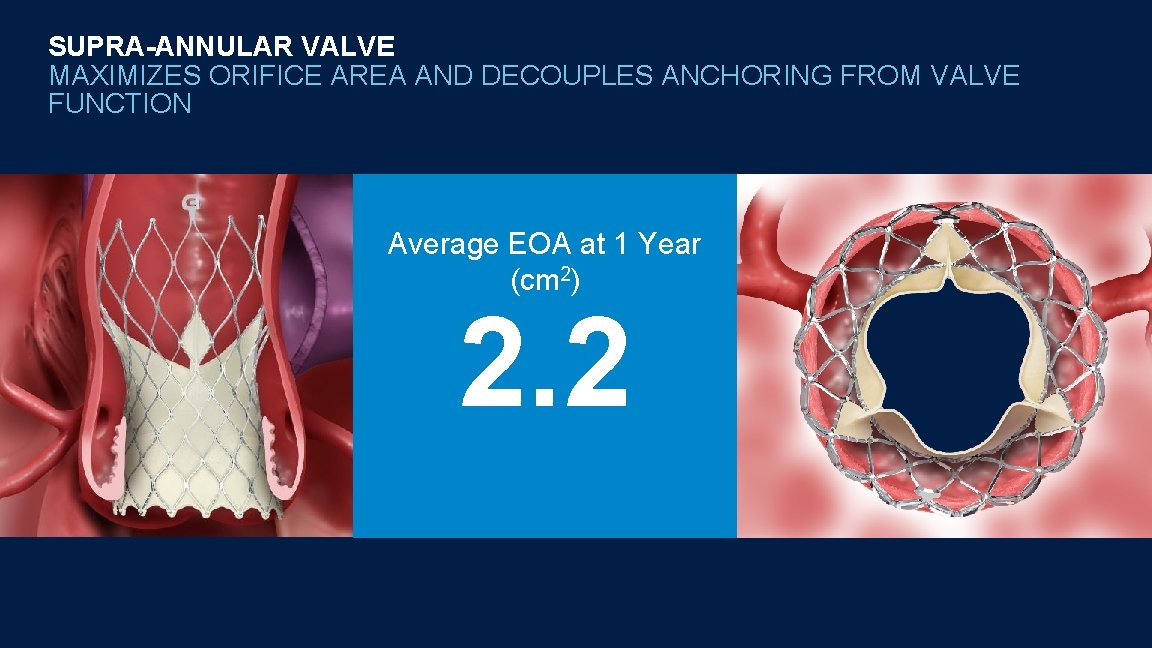
SUPRA-ANNULAR VALVE MAXIMIZES ORIFICE AREA AND DECOUPLES ANCHORING FROM VALVE FUNCTION Average EOA at 1 Year (cm 2) 2. 2

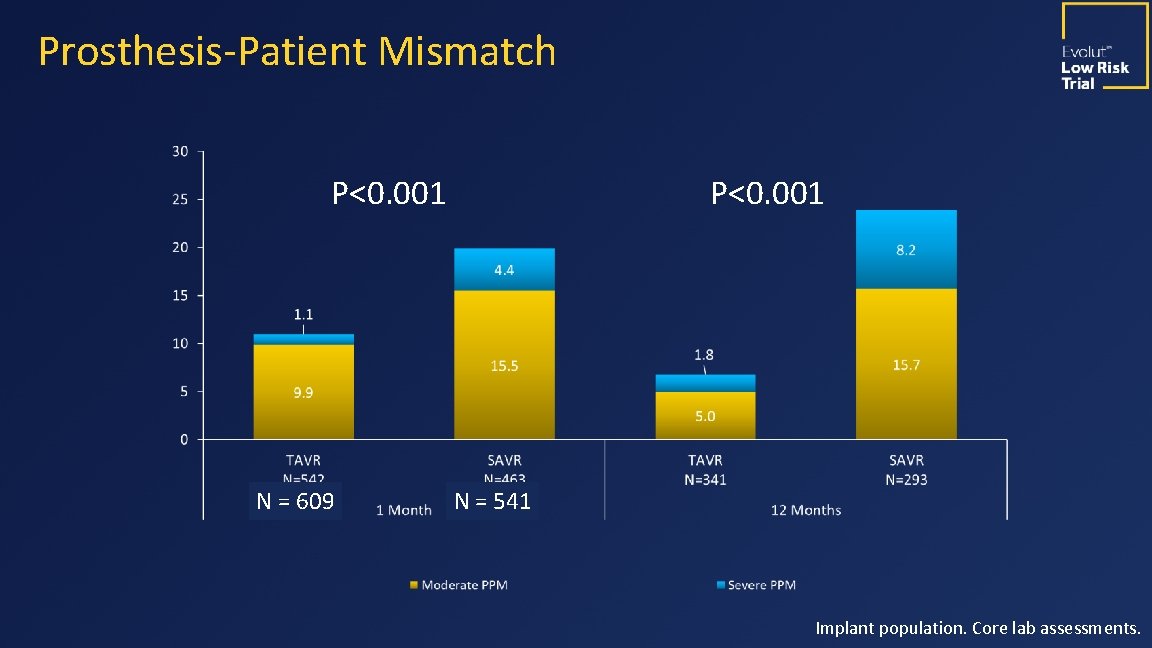
Prosthesis-Patient Mismatch P<0. 001 N = 609 P<0. 001 N = 541 Implant population. Core lab assessments.

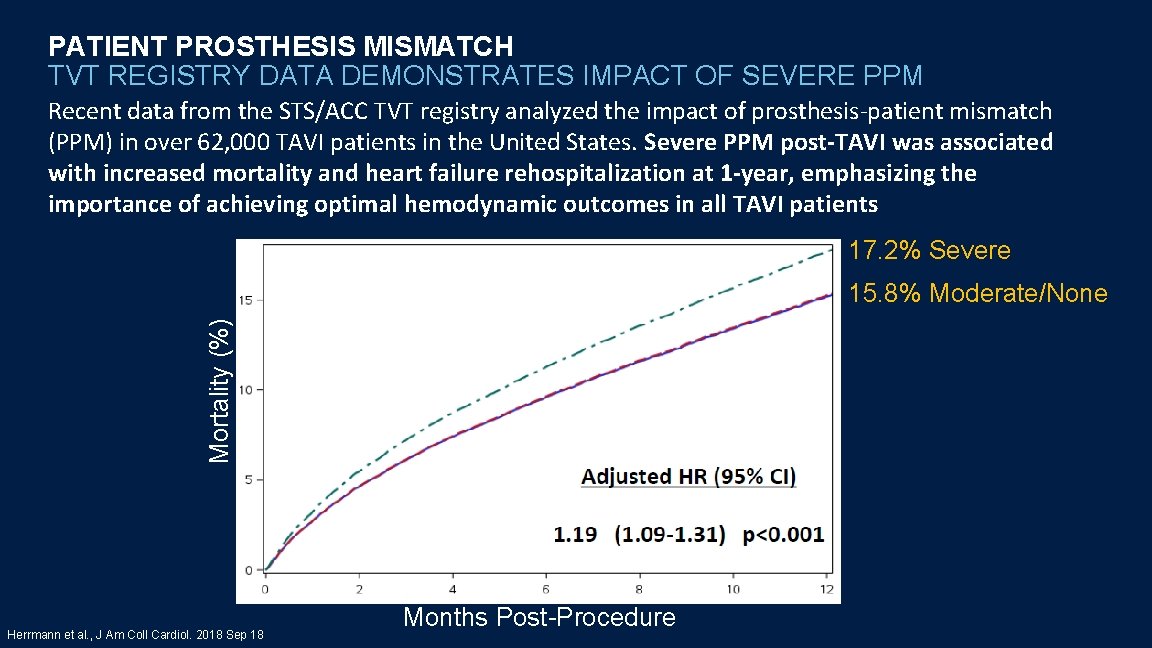
PATIENT PROSTHESIS MISMATCH TVT REGISTRY DATA DEMONSTRATES IMPACT OF SEVERE PPM Recent data from the STS/ACC TVT registry analyzed the impact of prosthesis-patient mismatch (PPM) in over 62, 000 TAVI patients in the United States. Severe PPM post-TAVI was associated with increased mortality and heart failure rehospitalization at 1 -year, emphasizing the importance of achieving optimal hemodynamic outcomes in all TAVI patients 17. 2% Severe Mortality (%) Mortality Herrmann et al. , J Am Coll Cardiol. 2018 Sep 18 Months Post-Procedure 15. 8% Moderate/None

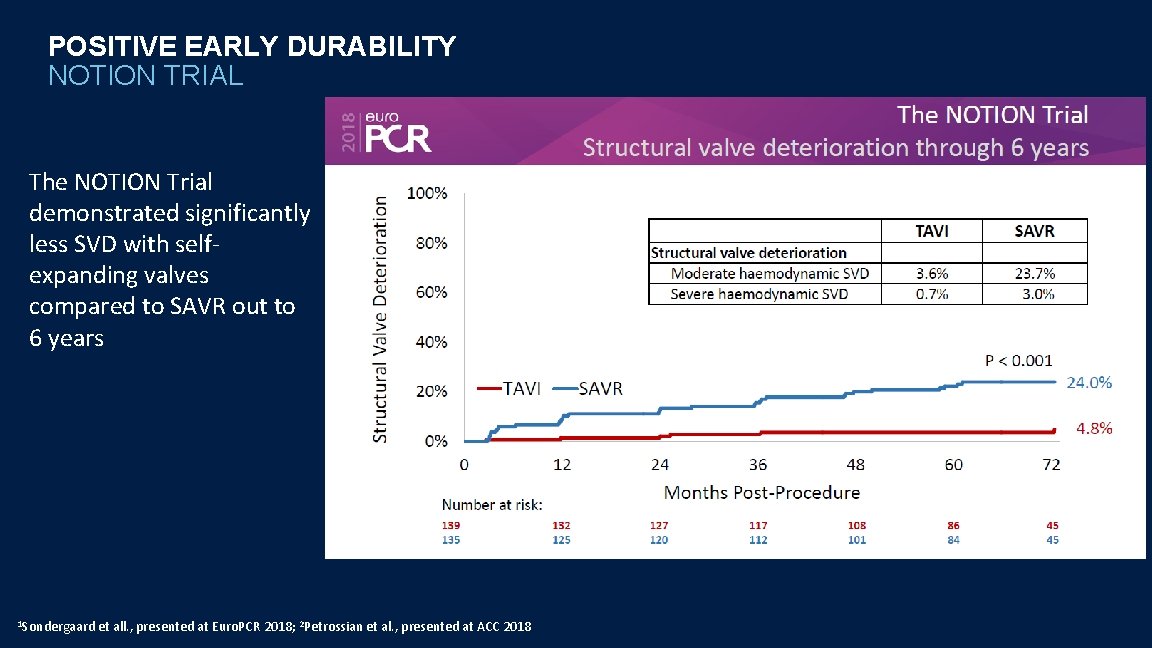
POSITIVE EARLY DURABILITY NOTION TRIAL The NOTION Trial demonstrated significantly less SVD with selfexpanding valves compared to SAVR out to 6 years 1 Sondergaard et all. , presented at Euro. PCR 2018; 2 Petrossian et al. , presented at ACC 2018

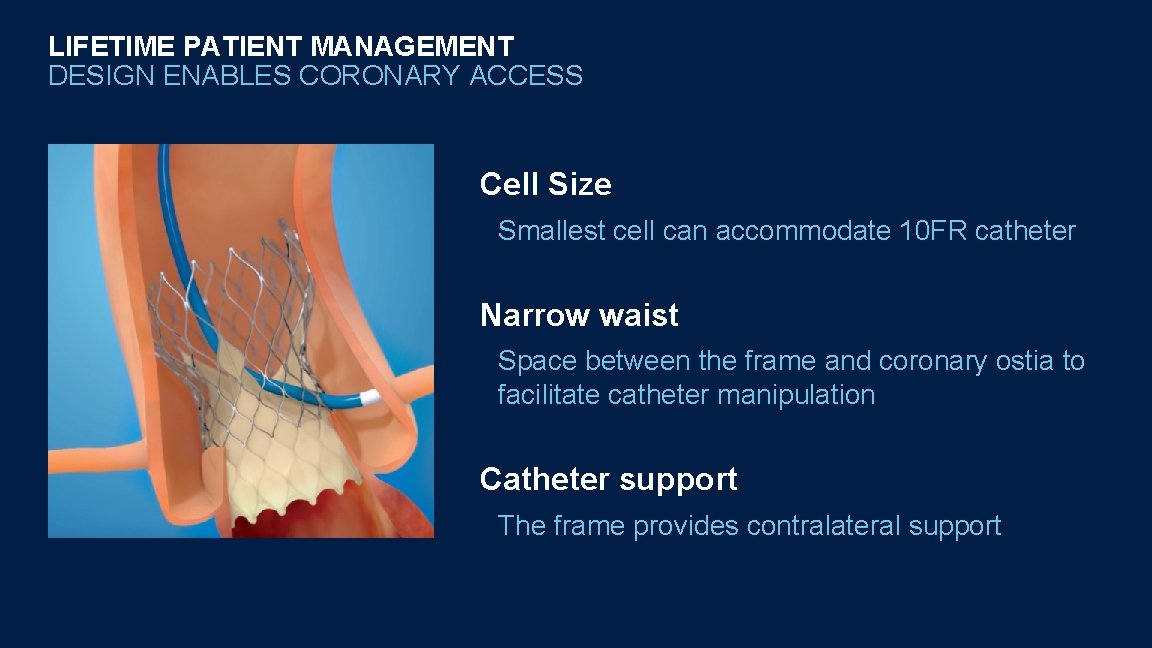
LIFETIME PATIENT MANAGEMENT DESIGN ENABLES CORONARY ACCESS Cell Size Smallest cell can accommodate 10 FR catheter Narrow waist Space between the frame and coronary ostia to facilitate catheter manipulation Catheter support The frame provides contralateral support

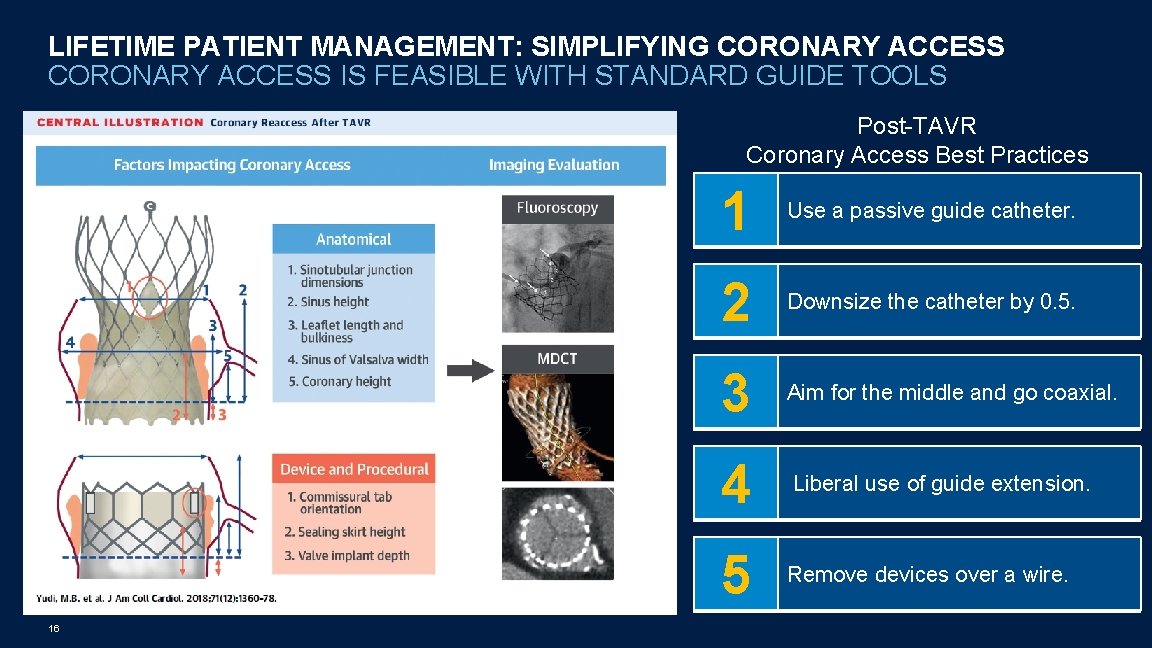
LIFETIME PATIENT MANAGEMENT: SIMPLIFYING CORONARY ACCESS IS FEASIBLE WITH STANDARD GUIDE TOOLS Post-TAVR Coronary Access Best Practices 16 1 Use a passive guide catheter. 2 Downsize the catheter by 0. 5. 3 Aim for the middle and go coaxial. 4 Liberal use of guide extension. 5 Remove devices over a wire.

FUTURE INNOVATION

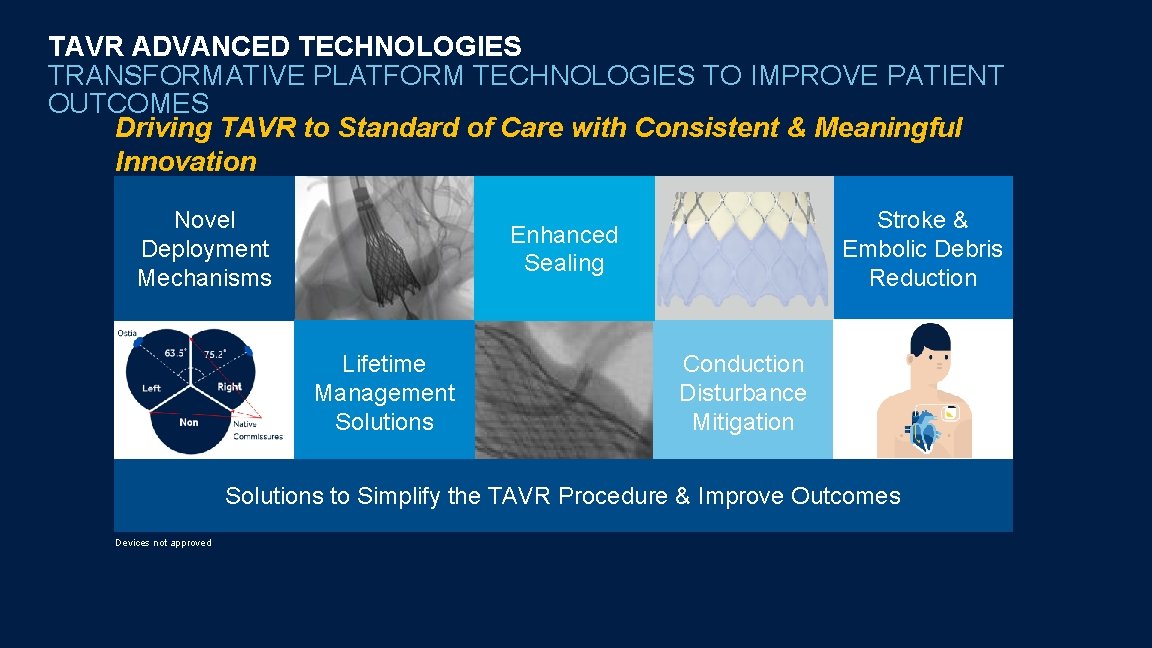
TAVR ADVANCED TECHNOLOGIES TRANSFORMATIVE PLATFORM TECHNOLOGIES TO IMPROVE PATIENT OUTCOMES Driving TAVR to Standard of Care with Consistent & Meaningful Innovation Novel Deployment Mechanisms Stroke & Embolic Debris Reduction Enhanced Sealing Lifetime Management Solutions Conduction Disturbance Mitigation Solutions to Simplify the TAVR Procedure & Improve Outcomes Devices not approved

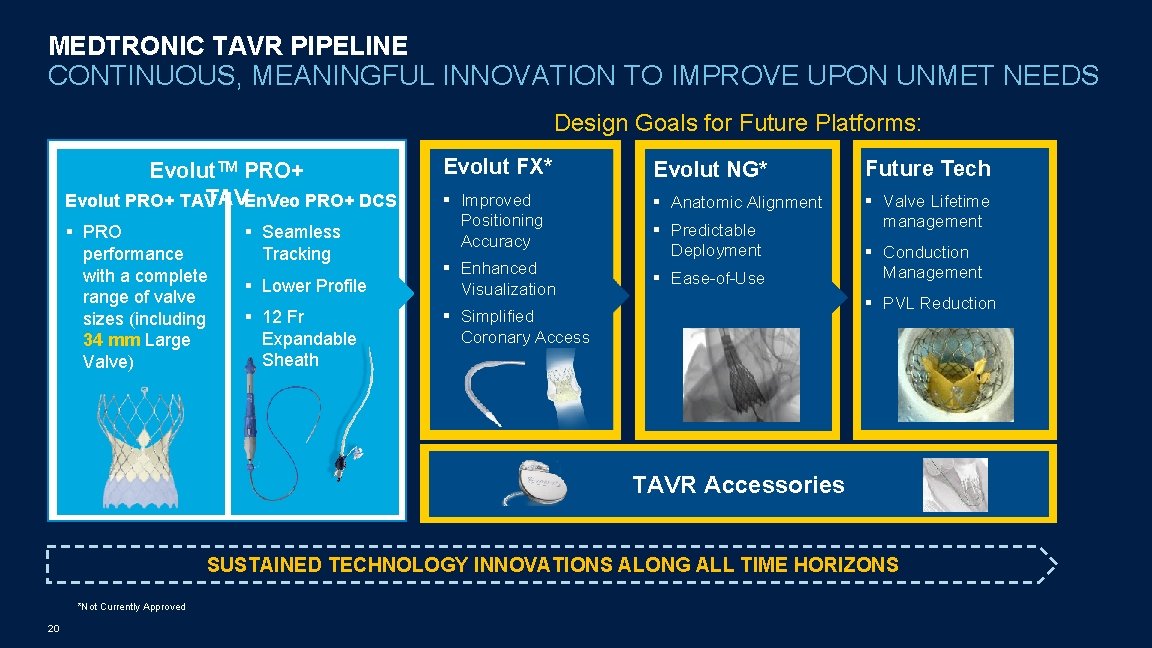
MEDTRONIC TAVR PIPELINE CONTINUOUS, MEANINGFUL INNOVATION TO IMPROVE UPON UNMET NEEDS Design Goals for Future Platforms: Evolut. TM PRO+ TAVEn. Veo PRO+ DCS Evolut PRO+ TAV § PRO performance with a complete range of valve sizes (including 34 mm Large Valve) § Seamless Tracking § Lower Profile § 12 Fr Expandable Sheath Evolut FX* Evolut NG* Future Tech § Improved Positioning Accuracy § Anatomic Alignment § Valve Lifetime management § Enhanced Visualization § Predictable Deployment § Ease-of-Use § Conduction Management § PVL Reduction § Simplified Coronary Access TAVR Accessories SUSTAINED TECHNOLOGY INNOVATIONS ALONG ALL TIME HORIZONS *Not Currently Approved 20

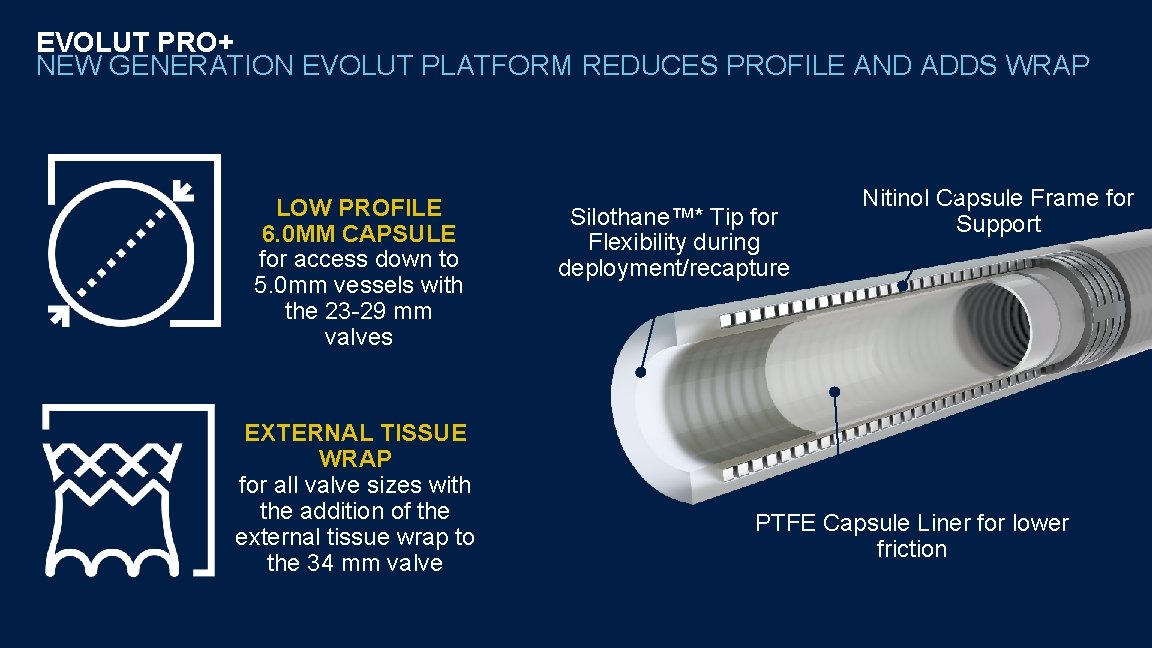
EVOLUT PRO+ NEW GENERATION EVOLUT PLATFORM REDUCES PROFILE AND ADDS WRAP LOW PROFILE 6. 0 MM CAPSULE for access down to 5. 0 mm vessels with the 23 -29 mm valves EXTERNAL TISSUE WRAP for all valve sizes with the addition of the external tissue wrap to the 34 mm valve Silothane™* Tip for Flexibility during deployment/recapture Nitinol Capsule Frame for Support PTFE Capsule Liner for lower friction

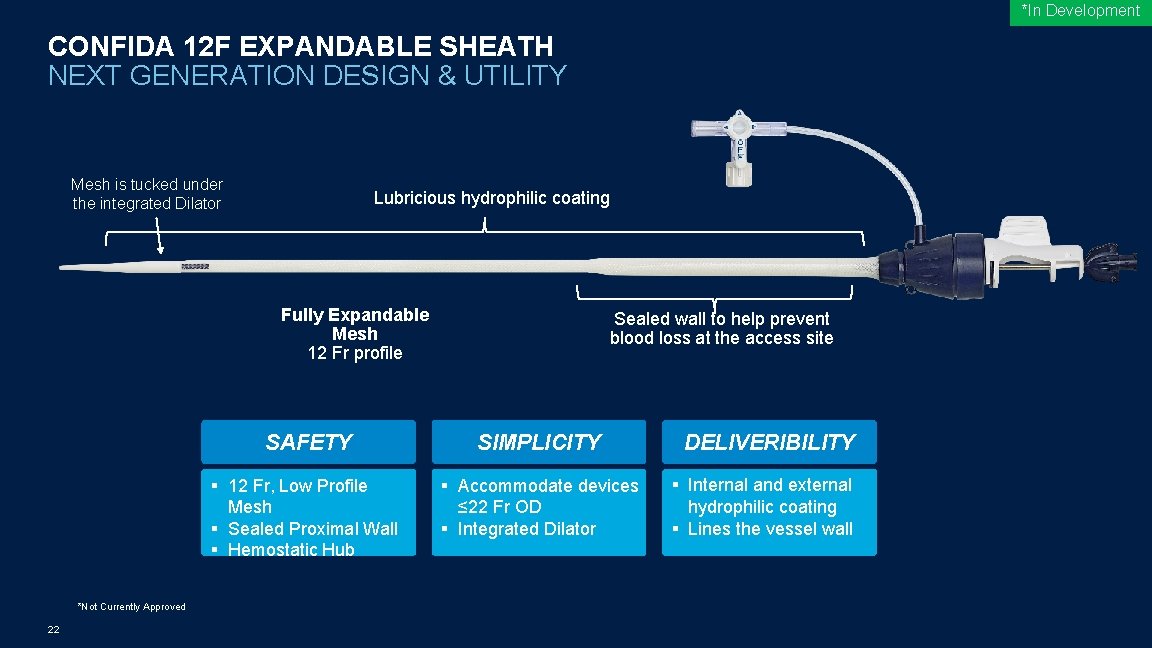
*In Development CONFIDA 12 F EXPANDABLE SHEATH NEXT GENERATION DESIGN & UTILITY Mesh is tucked under the integrated Dilator Radiopaque Dilator Tip *Not Currently Approved 22 Lubricious hydrophilic coating Fully Expandable Mesh 12 Fr profile Sealed wall to help prevent blood loss at the access site SAFETY SIMPLICITY § 12 Fr, Low Profile Mesh § Sealed Proximal Wall § Hemostatic Hub § Accommodate devices ≤ 22 Fr OD § Integrated Dilator DELIVERIBILITY § Internal and external hydrophilic coating § Lines the vessel wall

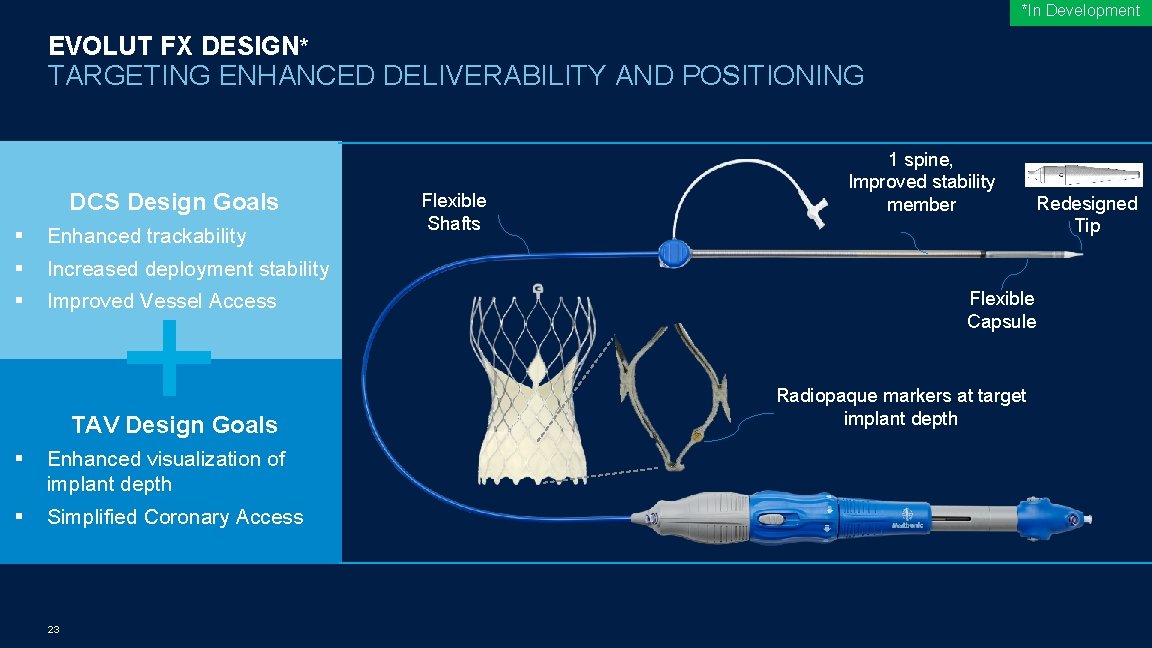
*In Development EVOLUT FX DESIGN* TARGETING ENHANCED DELIVERABILITY AND POSITIONING DCS Design Goals § Enhanced trackability § Increased deployment stability § Improved Vessel Access TAV Design Goals § Enhanced visualization of implant depth § Simplified Coronary Access 23 Flexible Shafts 1 spine, Improved stability member Redesigned Tip Flexible Capsule Radiopaque markers at target implant depth

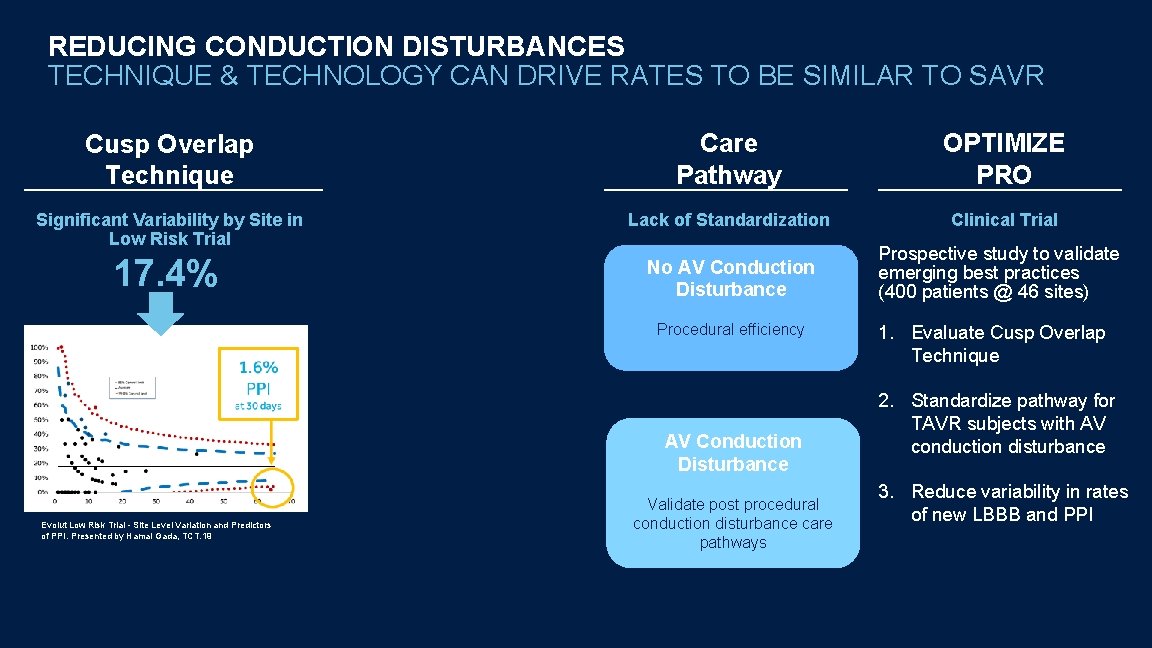
REDUCING CONDUCTION DISTURBANCES TECHNIQUE & TECHNOLOGY CAN DRIVE RATES TO BE SIMILAR TO SAVR Cusp Overlap Technique Care Pathway OPTIMIZE PRO Significant Variability by Site in Low Risk Trial Lack of Standardization Clinical Trial 17. 4% No AV Conduction Disturbance Prospective study to validate emerging best practices (400 patients @ 46 sites) Procedural efficiency AV Conduction Disturbance Evolut Low Risk Trial - Site Level Variation and Predictors of PPI. Presented by Hamal Gada, TCT. 19 Validate post procedural conduction disturbance care pathways 1. Evaluate Cusp Overlap Technique 2. Standardize pathway for TAVR subjects with AV conduction disturbance 3. Reduce variability in rates of new LBBB and PPI

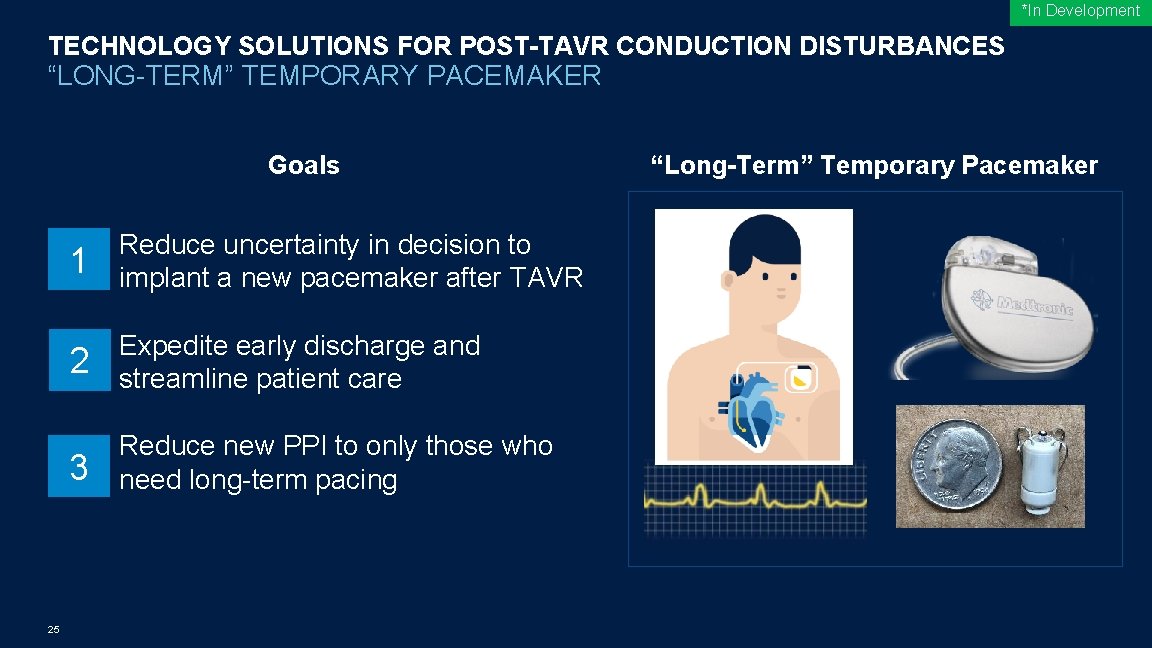
*In Development TECHNOLOGY SOLUTIONS FOR POST-TAVR CONDUCTION DISTURBANCES “LONG-TERM” TEMPORARY PACEMAKER Goals 25 1 Reduce uncertainty in decision to implant a new pacemaker after TAVR 2 Expedite early discharge and streamline patient care 3 Reduce new PPI to only those who need long-term pacing “Long-Term” Temporary Pacemaker

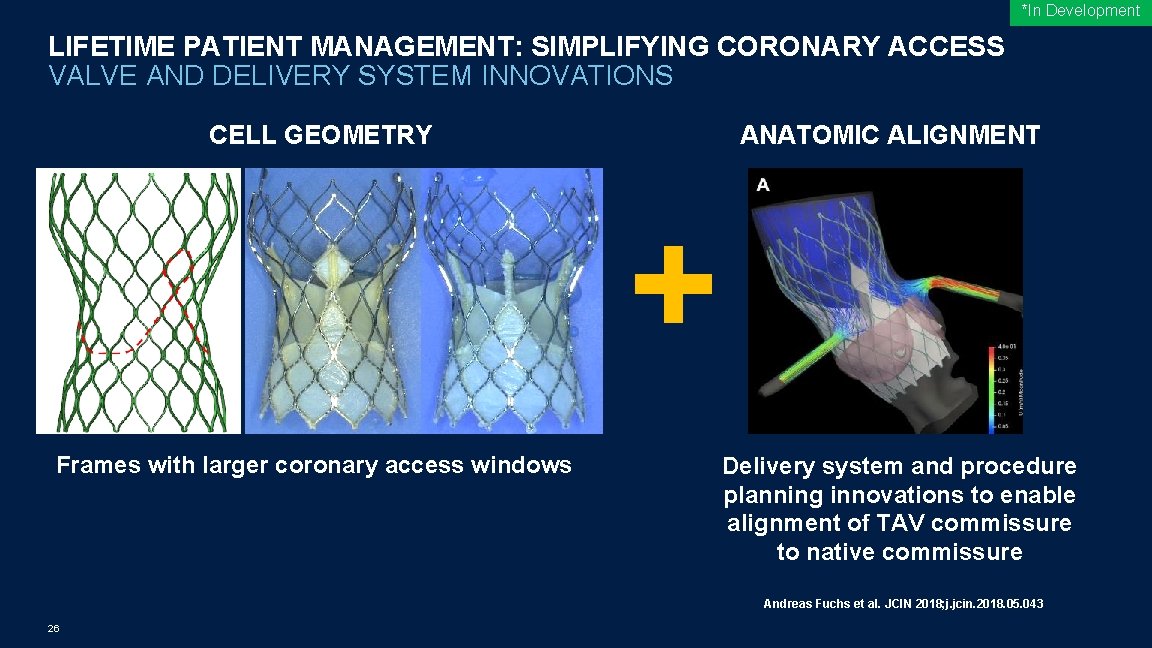
*In Development LIFETIME PATIENT MANAGEMENT: SIMPLIFYING CORONARY ACCESS VALVE AND DELIVERY SYSTEM INNOVATIONS CELL GEOMETRY Frames with larger coronary access windows ANATOMIC ALIGNMENT Delivery system and procedure planning innovations to enable alignment of TAV commissure to native commissure Andreas Fuchs et al. JCIN 2018; j. jcin. 2018. 05. 043 26

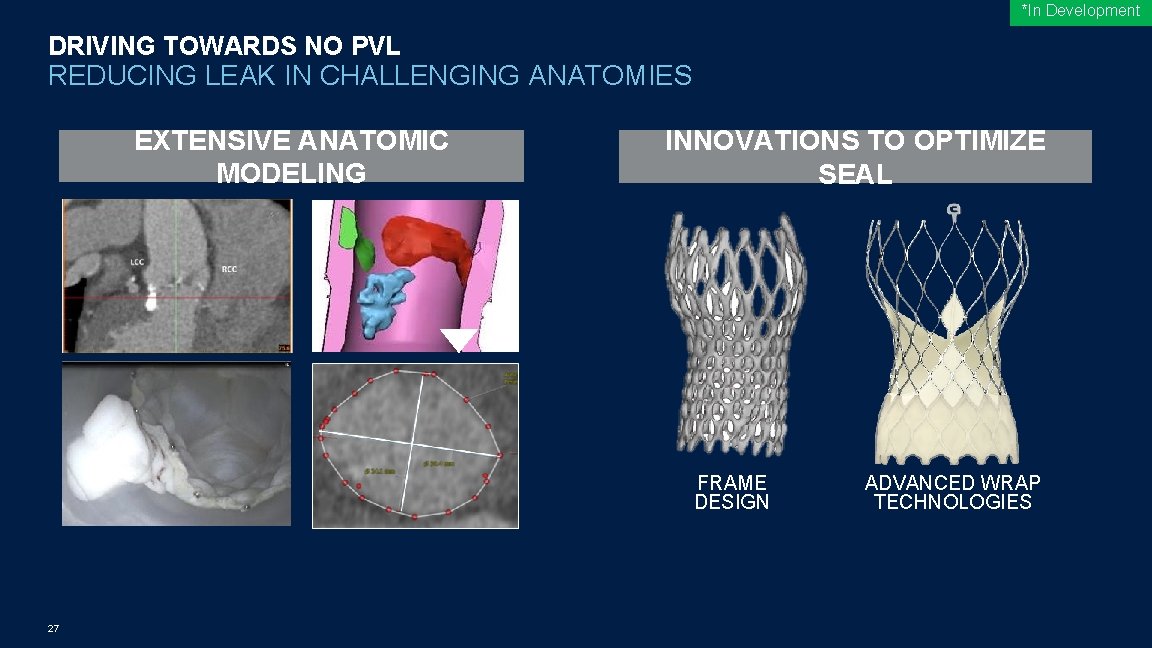
*In Development DRIVING TOWARDS NO PVL REDUCING LEAK IN CHALLENGING ANATOMIES EXTENSIVE ANATOMIC MODELING INNOVATIONS TO OPTIMIZE SEAL FRAME DESIGN 27 ADVANCED WRAP TECHNOLOGIES

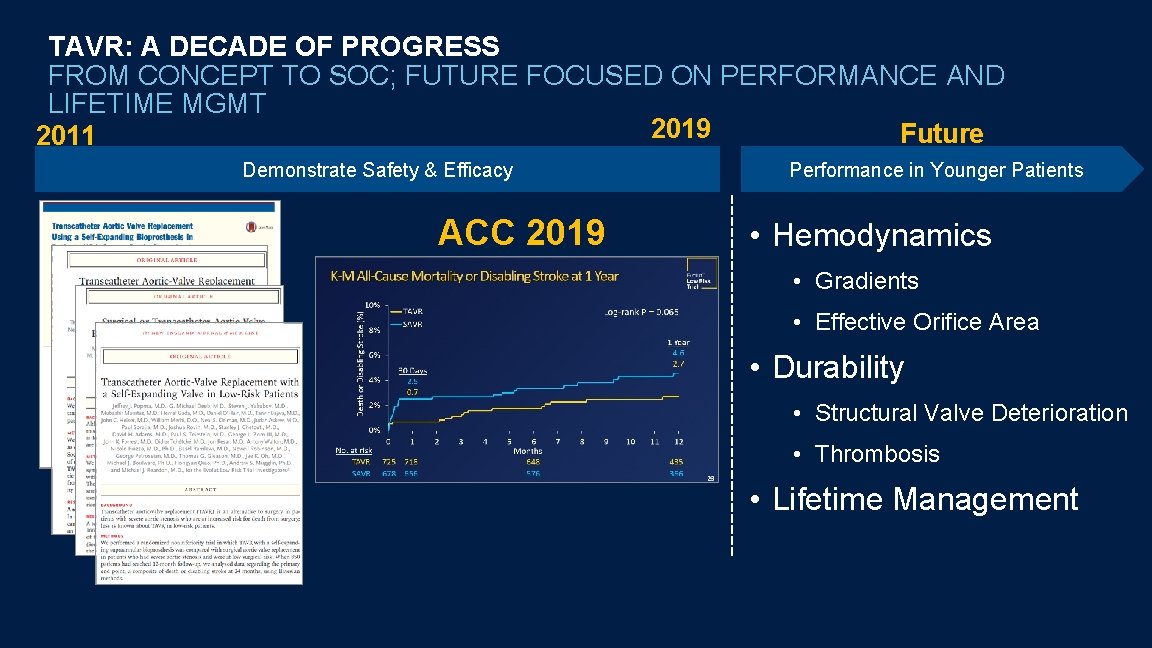
TAVR: A DECADE OF PROGRESS FROM CONCEPT TO SOC; FUTURE FOCUSED ON PERFORMANCE AND LIFETIME MGMT 2019 Future 2011 Demonstrate Safety & Efficacy ACC 2019 Performance in Younger Patients • Hemodynamics • Gradients • Effective Orifice Area • Durability • Structural Valve Deterioration • Thrombosis • Lifetime Management

THANK YOU