Copyright 2004 Pearson Education Inc publishing as Benjamin

Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

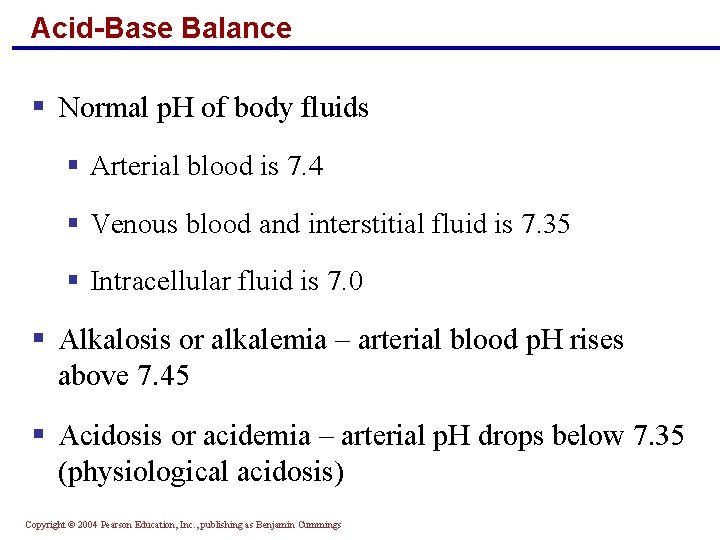
Acid-Base Balance § Normal p. H of body fluids § Arterial blood is 7. 4 § Venous blood and interstitial fluid is 7. 35 § Intracellular fluid is 7. 0 § Alkalosis or alkalemia – arterial blood p. H rises above 7. 45 § Acidosis or acidemia – arterial p. H drops below 7. 35 (physiological acidosis) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
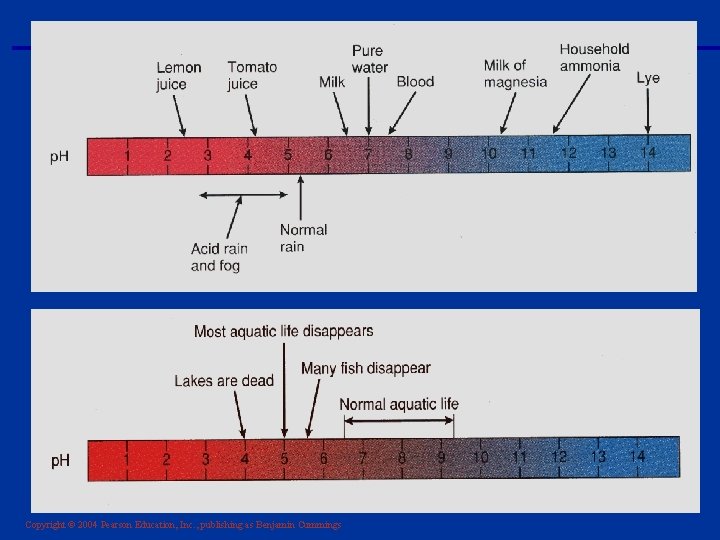
Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
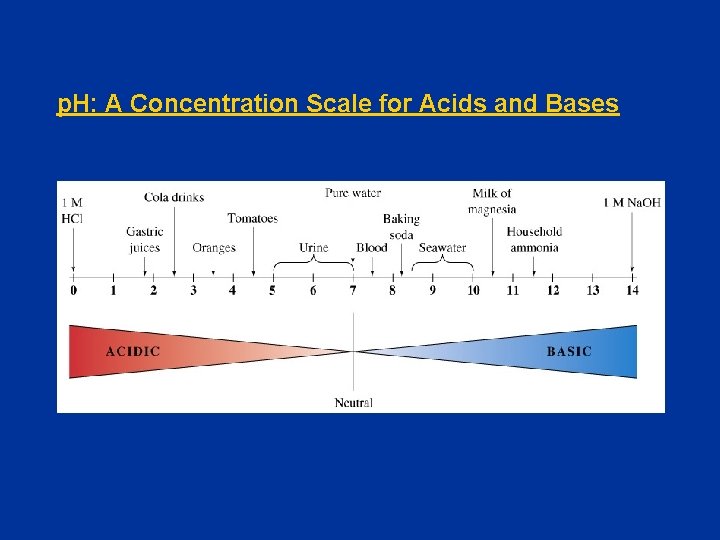
p. H: A Concentration Scale for Acids and Bases
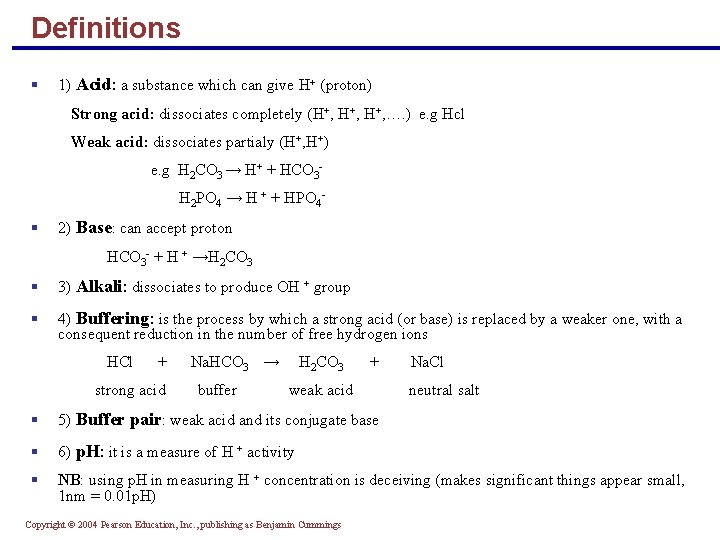
Definitions § 1) Acid: a substance which can give H+ (proton) Strong acid: dissociates completely (H+, H+, …. ) e. g Hcl Weak acid: dissociates partialy (H+, H+) e. g H 2 CO 3 → H+ + HCO 3 H 2 PO 4 → H + + HPO 4 - § 2) Base: can accept proton HCO 3 - + H + →H 2 CO 3 § 3) Alkali: dissociates to produce OH + group § 4) Buffering: is the process by which a strong acid (or base) is replaced by a weaker one, with a consequent reduction in the number of free hydrogen ions HCl + strong acid Na. HCO 3 → buffer H 2 CO 3 + weak acid Na. Cl neutral salt § 5) Buffer pair: weak acid and its conjugate base § 6) p. H: it is a measure of H + activity § NB: using p. H in measuring H + concentration is deceiving (makes significant things appear small, 1 nm = 0. 01 p. H) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Sources of Hydrogen Ions § Most hydrogen ions originate from cellular metabolism § Breakdown of phosphorus-containing proteins releases phosphoric acid into the ECF § Anaerobic respiration of glucose produces lactic acid § Fat metabolism yields organic acids and ketone bodies (ketoacids) § Transporting carbon dioxide as bicarbonate releases hydrogen ions Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Hydrogen Ion Regulation § Concentration of hydrogen ions is regulated sequentially by: § Chemical buffer systems – act within seconds § The respiratory center in the brain stem – acts within 1 -3 minutes § Renal mechanisms – require hours to days to effect p. H changes Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
A. Chemical Buffer Systems § Strong acids – all their H+ is dissociated completely in water § Weak acids – dissociate partially in water and are efficient at preventing p. H changes § Strong bases – dissociate easily in water and quickly tie up H+ § Weak bases – accept H+ more slowly (e. g. , HCO 3¯ and NH 3 ) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Chemical Buffer Systems § One or two molecules that act to resist p. H changes when strong acid or base is added § Three major chemical buffer systems § Bicarbonate buffer system § Phosphate buffer system § Protein buffer system § Any drifts in p. H are resisted by the entire chemical buffering system Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
1. Bicarbonate Buffer System § A mixture of carbonic acid (H 2 CO 3) and its salt, sodium bicarbonate (Na. HCO 3) (potassium or magnesium bicarbonates work as well) § If strong acid is added: § Hydrogen ions released combine with the bicarbonate ions and form carbonic acid (a weak acid) § The p. H of the solution decreases only slightly Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Bicarbonate Buffer System § If strong base is added: § It reacts with the carbonic acid to form sodium bicarbonate (a weak base) § The p. H of the solution rises only slightly § This system is the only important ECF buffer Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
2. Phosphate Buffer System § Nearly identical to the bicarbonate system § Its components are: § Sodium salts of dihydrogen phosphate (H 2 PO 4¯), a weak acid § Monohydrogen phosphate (HPO 42¯), a weak base § This system is an effective buffer in urine and intracellular fluid Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
3. Protein Buffer System § Plasma and intracellular proteins are the body’s most plentiful and powerful buffers § Some amino acids of proteins have: § Free organic acid groups (weak acids) § Groups that act as weak bases (e. g. , amino groups) § Amphoteric molecules are protein molecules that can function as both a weak acid and a weak base Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
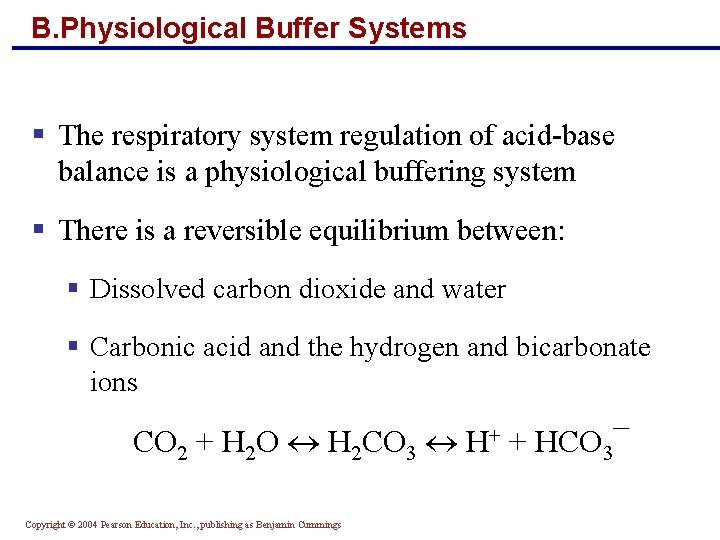
B. Physiological Buffer Systems § The respiratory system regulation of acid-base balance is a physiological buffering system § There is a reversible equilibrium between: § Dissolved carbon dioxide and water § Carbonic acid and the hydrogen and bicarbonate ions CO 2 + H 2 O H 2 CO 3 H+ + HCO 3¯ Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Physiological Buffer Systems § During carbon dioxide unloading, hydrogen ions are incorporated into water § When hypercapnia or rising plasma H+ occurs: § Deeper and more rapid breathing expels more carbon dioxide § Hydrogen ion concentration is reduced § Alkalosis causes slower, more shallow breathing, causing H+ to increase § Respiratory system impairment causes acid-base imbalance (respiratory acidosis or respiratory alkalosis) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
C. Renal Mechanisms of Acid-Base Balance § Chemical buffers can tie up excess acids or bases, but they cannot eliminate them from the body § The lungs can eliminate carbonic acid by eliminating carbon dioxide § Only the kidneys can rid the body of metabolic acids (phosphoric, uric, and lactic acids and ketones) and prevent metabolic acidosis § The ultimate acid-base regulatory organs are the kidneys Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Renal Mechanisms of Acid-Base Balance § The most important renal mechanisms for regulating acid-base balance are: § Conserving (reabsorbing) or generating new bicarbonate ions in acidosis § Excreting bicarbonate ions in alkalosis § Losing a bicarbonate ion is the same as gaining a hydrogen ion; reabsorbing a bicarbonate ion is the same as losing a hydrogen ion Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
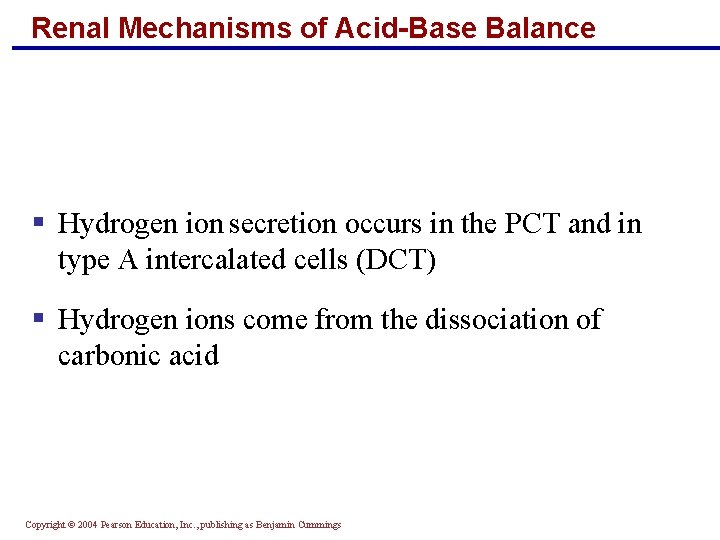
Renal Mechanisms of Acid-Base Balance § Hydrogen ion secretion occurs in the PCT and in type A intercalated cells (DCT) § Hydrogen ions come from the dissociation of carbonic acid Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Reabsorption of Bicarbonate § Carbon dioxide combines with water in tubule cells, forming carbonic acid § Carbonic acid splits into hydrogen ions and bicarbonate ions § For each hydrogen ion secreted, a sodium ion and a bicarbonate ion are reabsorbed by the PCT cells § Secreted hydrogen ions form carbonic acid; thus, bicarbonate disappears from filtrate at the same rate that it enters the peritubular capillary blood Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
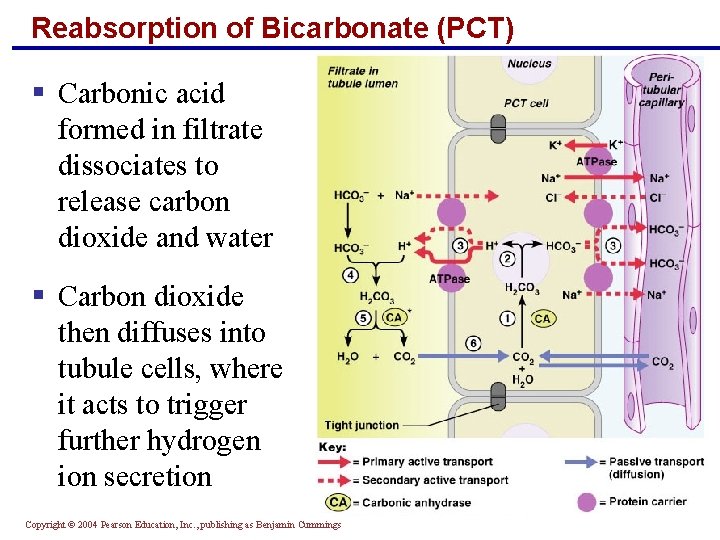
Reabsorption of Bicarbonate (PCT) § Carbonic acid formed in filtrate dissociates to release carbon dioxide and water § Carbon dioxide then diffuses into tubule cells, where it acts to trigger further hydrogen ion secretion Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Generating New Bicarbonate Ions (DCT) § Two mechanisms carried out by type A intercalated cells in DCT generate new bicarbonate ions § Both involve renal excretion of acid via secretion and excretion of hydrogen ions or ammonium ions (NH 4+) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Hydrogen Ion Excretion § Dietary hydrogen ions must be counteracted by generating new bicarbonate § The excreted hydrogen ions must bind to buffers in the urine (phosphate buffer system) § Intercalated cells actively secrete hydrogen ions into urine, which is buffered and excreted § Bicarbonate generated is: § Moved into the interstitial space via a cotransport system § Passively moved into the peritubular capillary blood Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
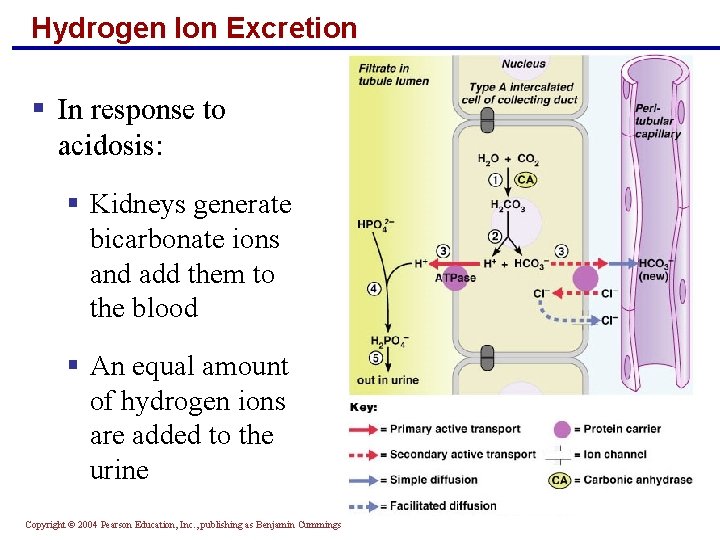
Hydrogen Ion Excretion § In response to acidosis: § Kidneys generate bicarbonate ions and add them to the blood § An equal amount of hydrogen ions are added to the urine Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Ammonium Ion Excretion § This method uses ammonium ions produced by the metabolism of glutamine in PCT cells § Each glutamine metabolized produces two ammonium ions and two bicarbonate ions § Bicarbonate moves to the blood and ammonium ions are excreted in urine Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
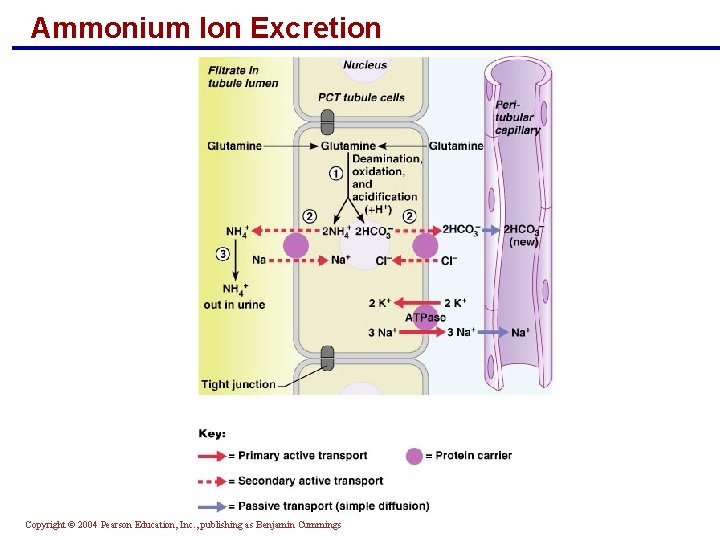
Ammonium Ion Excretion Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Bicarbonate Ion Secretion § When the body is in alkalosis, type B intercalated cells: § Exhibit bicarbonate ion secretion § Reclaim hydrogen ions and acidify the blood § The mechanism is the opposite of type A intercalated cells and the bicarbonate ion reabsorption process § Even during alkalosis, the nephrons and collecting ducts excrete fewer bicarbonate ions than they conserve Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Acid Base Dysequilibrium: § Metabolic acidosis § Metabolic alkalosis § Respiratory acidosis § Respiratory alkalosis § Mixed acid base disorder Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Respiratory acidosis and Alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Respiratory Acidosis and Alkalosis § Result from failure of the respiratory system to balance p. H § PCO 2 is the single most important indicator of respiratory inadequacy § PCO 2 levels § Normal PCO 2 fluctuates between 35 and 45 mm Hg § Values above 45 mm Hg signal respiratory acidosis § Values below 35 mm Hg indicate respiratory alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Respiratory Acidosis and Alkalosis § Respiratory acidosis is the most common cause of acid-base imbalance § Occurs when a person breathes shallowly (alveolar hypoventilation), or gas exchange is hampered (ventilation perfusion mismatch) by diseases such as pneumonia, cystic fibrosis, or emphysema § Respiratory alkalosis is a common result of hyperventilation § Occurs due to stimulation of respiratory center with cause other than hypercapnea Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
A. Respiratory Acidosis Differential diagnosis of respiratory acidosis: 1. Abnormal respiratory drive: - central alveolar hypoventilation - sleep apnea - brain stem infarction - myxoedema 2. Abnormal nerve conduction: - poliomyelitis - multiple sclerosis - amyotrophic lateral sclerosis - phrenic nerve injury - Guillian-Barre syndrome Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Respiratory Acidosis 3. Thoracic cage problems: - kyphoscoliosis - ankylosing spondylitis - muscular dystrophy - polymyositis 4. Pleural disease: - fibrothorax - hydrothorax 5. Pulmonary disease: - advanced interstitial pulmonary fibrosis - chronic obstructive lung disease Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

B. Respiratory Alkalosis § Causes: - mass lesions of the brain - inflammatory lesions of the brain - psychatric disorders - centrally acting drugs and chemicals - salicylates - endotoxins - progestrone - disorders that causes hypoxemia - disorders that decrease lung or chest compliance as pneumonia and pulmonary oedema - vascular cause as pulmonary emboli - hepatic disorders (or failure) - volume depletion → stimulus to hyperventilation and hypocapnea Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Metabolic Acidosis and Alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
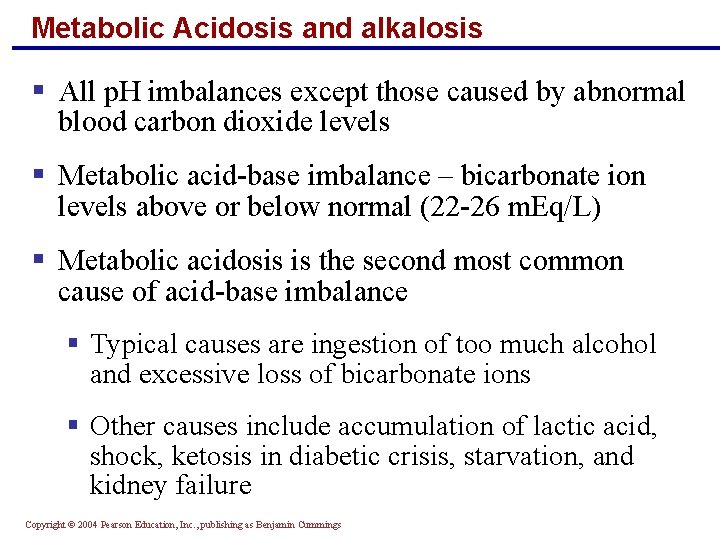
Metabolic Acidosis and alkalosis § All p. H imbalances except those caused by abnormal blood carbon dioxide levels § Metabolic acid-base imbalance – bicarbonate ion levels above or below normal (22 -26 m. Eq/L) § Metabolic acidosis is the second most common cause of acid-base imbalance § Typical causes are ingestion of too much alcohol and excessive loss of bicarbonate ions § Other causes include accumulation of lactic acid, shock, ketosis in diabetic crisis, starvation, and kidney failure Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
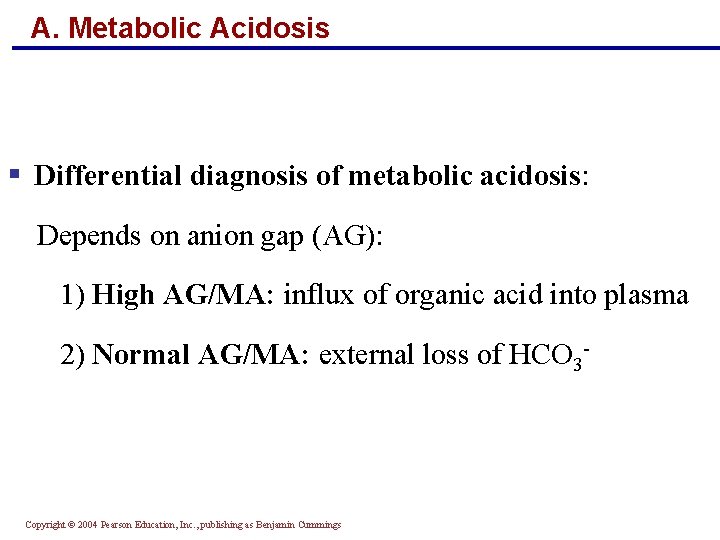
A. Metabolic Acidosis § Differential diagnosis of metabolic acidosis: Depends on anion gap (AG): 1) High AG/MA: influx of organic acid into plasma 2) Normal AG/MA: external loss of HCO 3 - Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
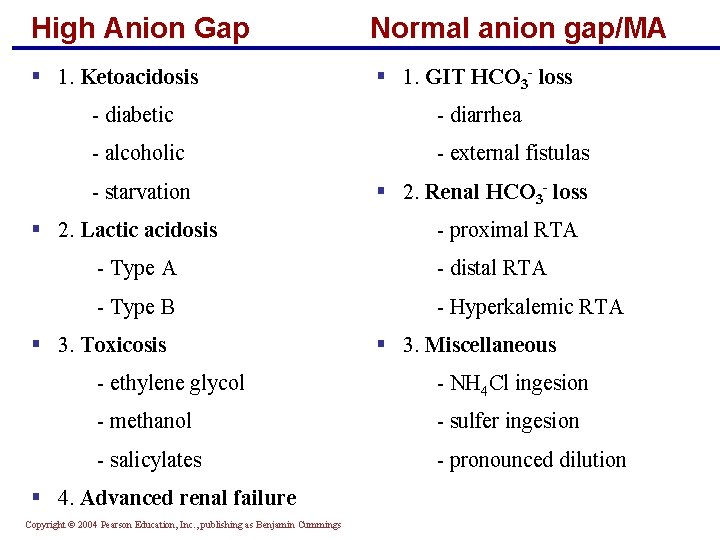
High Anion Gap Normal anion gap/MA § 1. Ketoacidosis § 1. GIT HCO 3 - loss - diabetic - diarrhea - alcoholic - external fistulas - starvation § 2. Renal HCO 3 - loss § 2. Lactic acidosis - proximal RTA - Type A - distal RTA - Type B - Hyperkalemic RTA § 3. Toxicosis § 3. Miscellaneous - ethylene glycol - NH 4 Cl ingesion - methanol - sulfer ingesion - salicylates - pronounced dilution § 4. Advanced renal failure Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

1. Ketoacidosis (KA) Arises when glucose is not available to cell due to: § Insulin lack (diabetic KA) § Cell dysfunction (alcoholic KA) § Glucose depletion (starvation KA) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

KA Fatty acids will be oxidized producing § Acetone (not an acid) § Acetoacetic acid § B-hydroxybuteric acid Plus § Energy Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
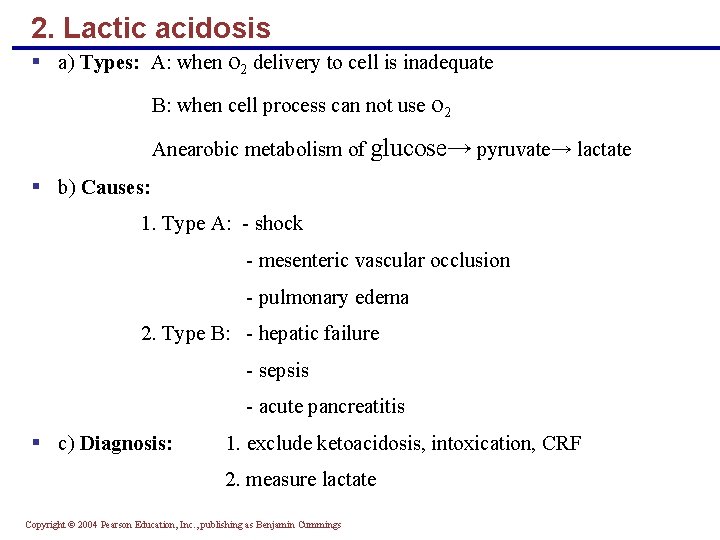
2. Lactic acidosis § a) Types: A: when o 2 delivery to cell is inadequate B: when cell process can not use o 2 Anearobic metabolism of glucose→ pyruvate→ lactate § b) Causes: 1. Type A: - shock - mesenteric vascular occlusion - pulmonary edema 2. Type B: - hepatic failure - sepsis - acute pancreatitis § c) Diagnosis: 1. exclude ketoacidosis, intoxication, CRF 2. measure lactate Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
3. Intoxication § Many substances can cause intoxication but most common examples are ethylene glycol, methanol (both are low MW alcohols which enters cells readily), and salicylates § They cause ↑AG/MA (but more lactic acidosis) § Metabolism: H+ → acidosis Methanol → formic acid Ethelyen glycol → glycolic acid + oxalate → needle like or envelop-shaped Ca-oxalate crystals in urine § Clues to diagnosis: ↑ alcohols in blood ↑ AG/Ma ↑ Osmaller gap (↑ 15 - 20 , difference between measured and calculated osmolality) calculated osmolality = 2 x Na+ + glucose/ 18 + BUN/2. 8 + ethanol/ 4. 6 § Salicylate intoxication → metabolic acidosis → respiratory alkalosis → mixed Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
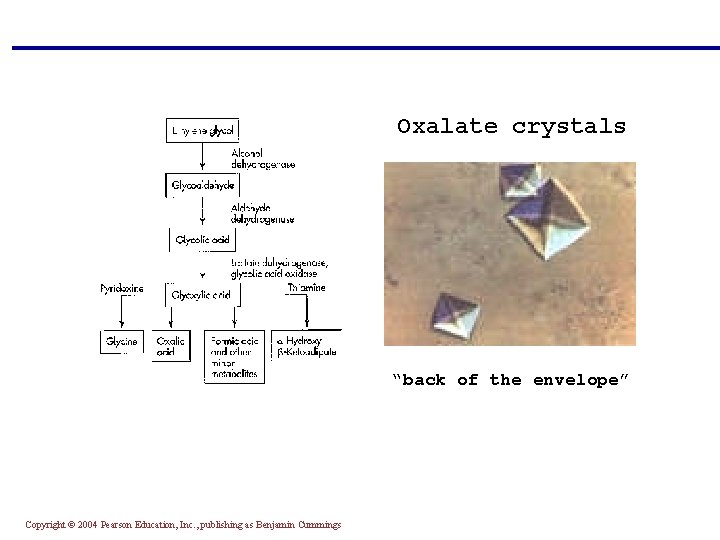
Oxalate crystals “back of the envelope” Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
4. Renal failure § In CRF → HCO 3 - ↑ 15 due to bone buffering § In ARF → HCO 3 - ↓ by 0. 5 m. Eq/L/day § Retention of sulfate and phosphate organic anions → ↑ AG/MA Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Normal AG/MA (Hyperchloremic ) § Causes: - GIT HCO 3 - loss - Renal HCO 3 - loss - Inorganic acid intake 1. GIT loss of HCO 3 -: GIT distal to the stomach is chloride absorbing HCO 3 - secreting Examples: * Diarrhea * Drainage : - pancreatic juice - biliary juice - small bowel juice * Ureterosigmoidostomy Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
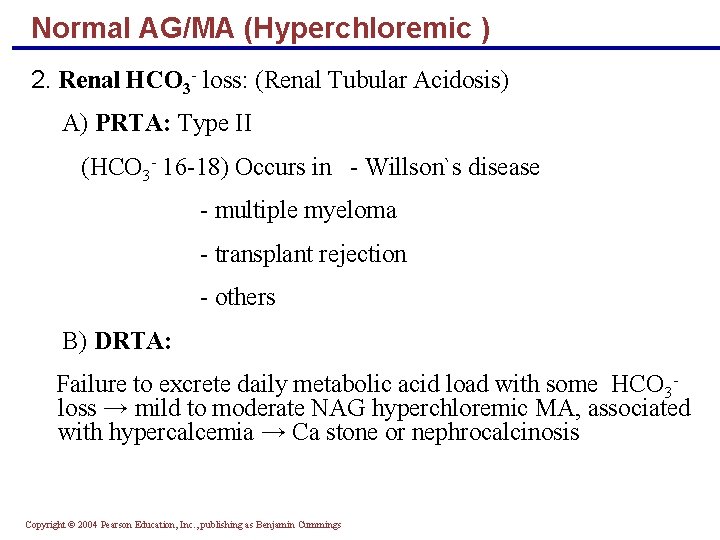
Normal AG/MA (Hyperchloremic ) 2. Renal HCO 3 - loss: (Renal Tubular Acidosis) A) PRTA: Type II (HCO 3 - 16 -18) Occurs in - Willson`s disease - multiple myeloma - transplant rejection - others B) DRTA: Failure to excrete daily metabolic acid load with some HCO 3 loss → mild to moderate NAG hyperchloremic MA, associated with hypercalcemia → Ca stone or nephrocalcinosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
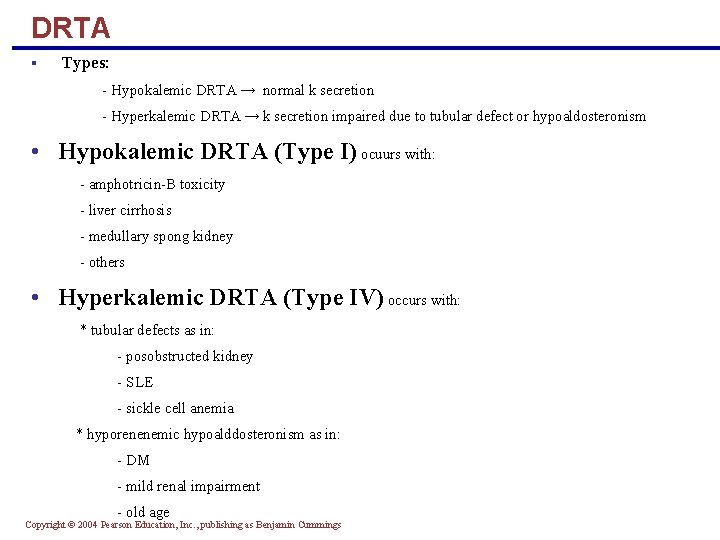
DRTA § Types: - Hypokalemic DRTA → normal k secretion - Hyperkalemic DRTA → k secretion impaired due to tubular defect or hypoaldosteronism • Hypokalemic DRTA (Type I) ocuurs with: - amphotricin-B toxicity - liver cirrhosis - medullary spong kidney - others • Hyperkalemic DRTA (Type IV) occurs with: * tubular defects as in: - posobstructed kidney - SLE - sickle cell anemia * hyporenenemic hypoalddosteronism as in: - DM - mild renal impairment - old age Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
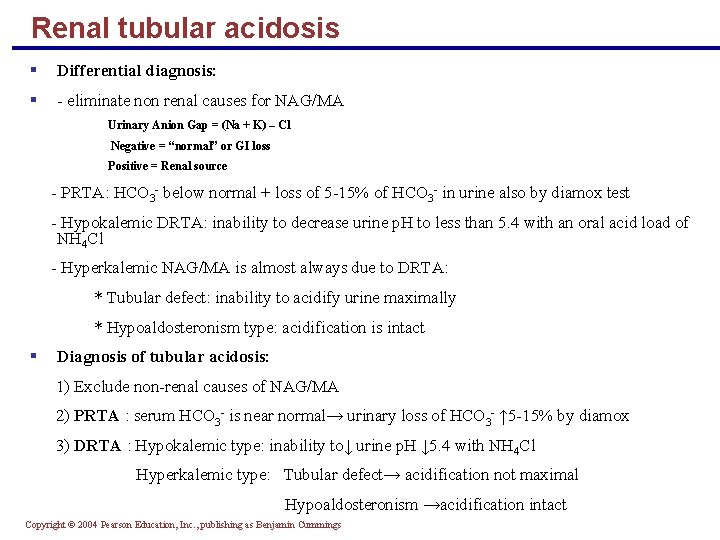
Renal tubular acidosis § Differential diagnosis: § - eliminate non renal causes for NAG/MA Urinary Anion Gap = (Na + K) – Cl Negative = “normal” or GI loss Positive = Renal source - PRTA: HCO 3 - below normal + loss of 5 -15% of HCO 3 - in urine also by diamox test - Hypokalemic DRTA: inability to decrease urine p. H to less than 5. 4 with an oral acid load of NH 4 Cl - Hyperkalemic NAG/MA is almost always due to DRTA: * Tubular defect: inability to acidify urine maximally * Hypoaldosteronism type: acidification is intact § Diagnosis of tubular acidosis: 1) Exclude non-renal causes of NAG/MA 2) PRTA : serum HCO 3 - is near normal→ urinary loss of HCO 3 - ↑ 5 -15% by diamox 3) DRTA : Hypokalemic type: inability to↓ urine p. H ↓ 5. 4 with NH 4 Cl Hyperkalemic type: Tubular defect→ acidification not maximal Hypoaldosteronism →acidification intact Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
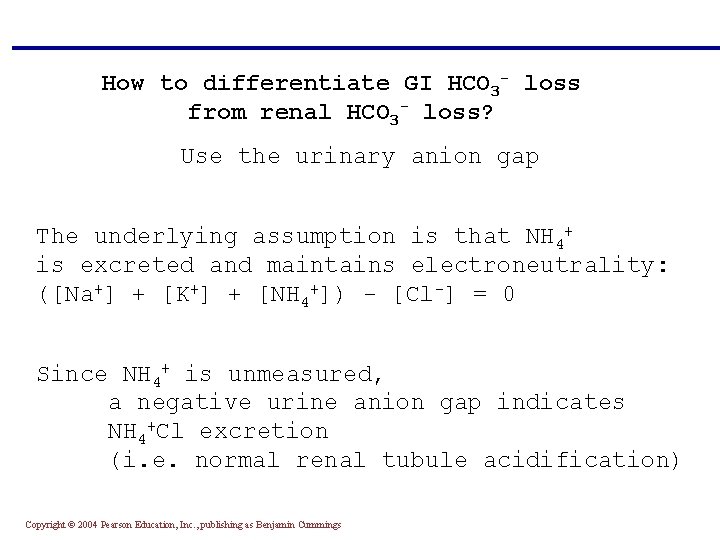
How to differentiate GI HCO 3 - loss from renal HCO 3 - loss? Use the urinary anion gap The underlying assumption is that NH 4+ is excreted and maintains electroneutrality: ([Na+] + [K+] + [NH 4+]) - [Cl-] = 0 Since NH 4+ is unmeasured, a negative urine anion gap indicates NH 4+Cl excretion (i. e. normal renal tubule acidification) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
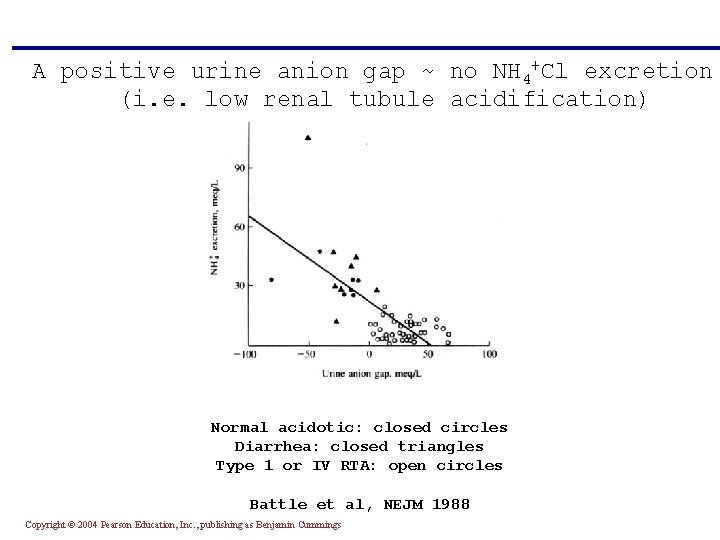
A positive urine anion gap ~ no NH 4+Cl excretion (i. e. low renal tubule acidification) Normal acidotic: closed circles Diarrhea: closed triangles Type 1 or IV RTA: open circles Battle et al, NEJM 1988 Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Metabolic Alkalosis § Rising blood p. H and bicarbonate levels indicate metabolic alkalosis § Typical causes are: § Vomiting of the acid contents of the stomach § Intake of excess base (e. g. , from antacids) § Constipation, in which excessive bicarbonate is reabsorbed Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
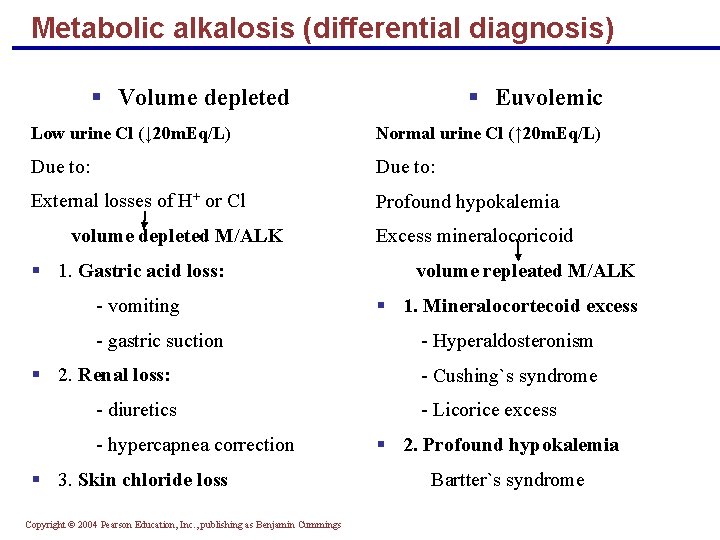
Metabolic alkalosis (differential diagnosis) § Volume depleted § Euvolemic Low urine Cl (↓ 20 m. Eq/L) Normal urine Cl (↑ 20 m. Eq/L) Due to: External losses of H+ or Cl Profound hypokalemia volume depleted M/ALK § 1. Gastric acid loss: - vomiting - gastric suction § 2. Renal loss: - diuretics - hypercapnea correction § 3. Skin chloride loss Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings Excess mineralocoricoid volume repleated M/ALK § 1. Mineralocortecoid excess - Hyperaldosteronism - Cushing`s syndrome - Licorice excess § 2. Profound hypokalemia Bartter`s syndrome
Respiratory and Renal Compensations § Acid-base imbalance due to inadequacy of a physiological buffer system is compensated for by the other system § The respiratory system will attempt to correct metabolic acid-base imbalances § The kidneys will work to correct imbalances caused by respiratory disease Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
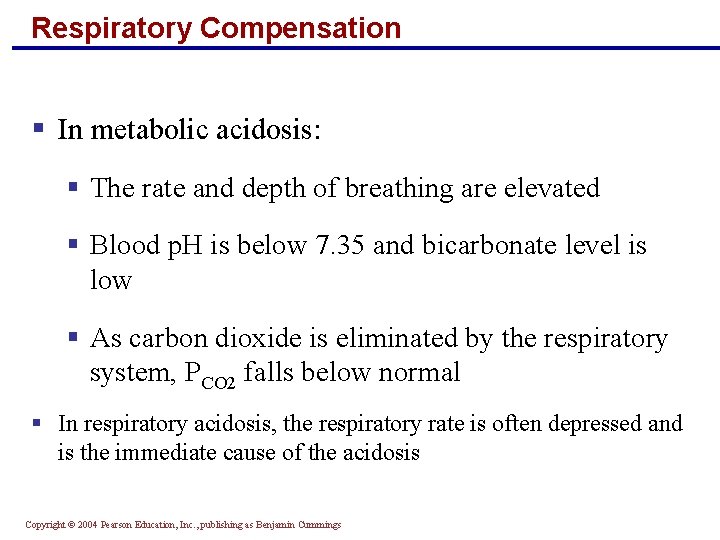
Respiratory Compensation § In metabolic acidosis: § The rate and depth of breathing are elevated § Blood p. H is below 7. 35 and bicarbonate level is low § As carbon dioxide is eliminated by the respiratory system, PCO 2 falls below normal § In respiratory acidosis, the respiratory rate is often depressed and is the immediate cause of the acidosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
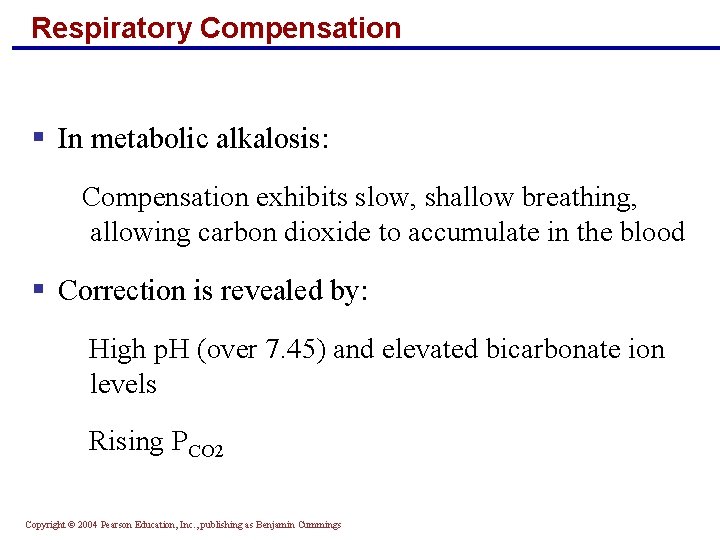
Respiratory Compensation § In metabolic alkalosis: Compensation exhibits slow, shallow breathing, allowing carbon dioxide to accumulate in the blood § Correction is revealed by: High p. H (over 7. 45) and elevated bicarbonate ion levels Rising PCO 2 Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
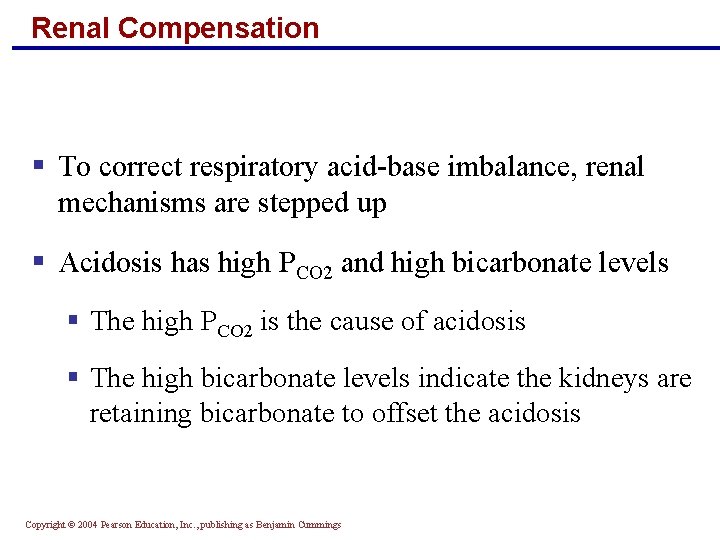
Renal Compensation § To correct respiratory acid-base imbalance, renal mechanisms are stepped up § Acidosis has high PCO 2 and high bicarbonate levels § The high PCO 2 is the cause of acidosis § The high bicarbonate levels indicate the kidneys are retaining bicarbonate to offset the acidosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
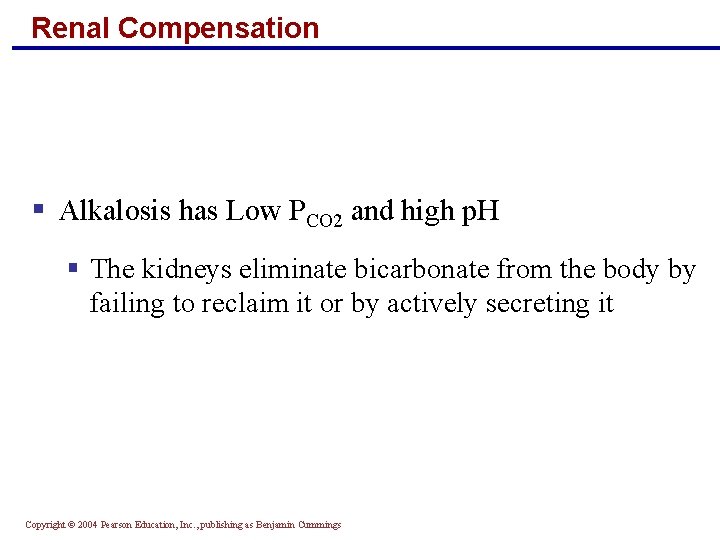
Renal Compensation § Alkalosis has Low PCO 2 and high p. H § The kidneys eliminate bicarbonate from the body by failing to reclaim it or by actively secreting it Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
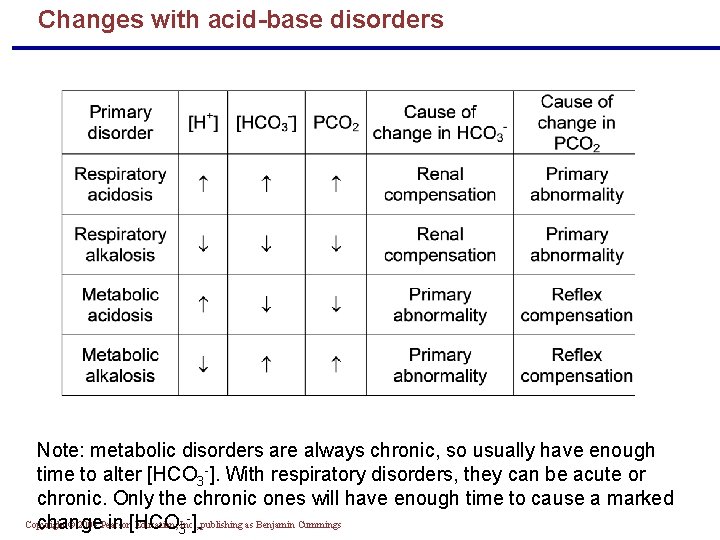
Changes with acid-base disorders Note: metabolic disorders are always chronic, so usually have enough time to alter [HCO 3 -]. With respiratory disorders, they can be acute or chronic. Only the chronic ones will have enough time to cause a marked Copyright © 2004 Pearson Education, Inc. , change in [HCO 3 ]. publishing as Benjamin Cummings
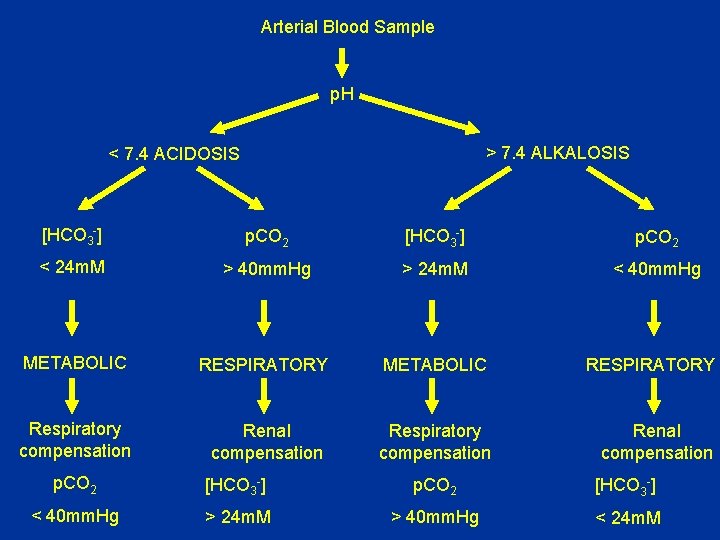
Arterial Blood Sample p. H > 7. 4 ALKALOSIS < 7. 4 ACIDOSIS [HCO 3 -] p. CO 2 < 24 m. M > 40 mm. Hg > 24 m. M < 40 mm. Hg METABOLIC RESPIRATORY Respiratory compensation Renal compensation p. CO 2 [HCO 3 -] < 40 mm. Hg > 24 m. M > 40 mm. Hg < 24 m. M
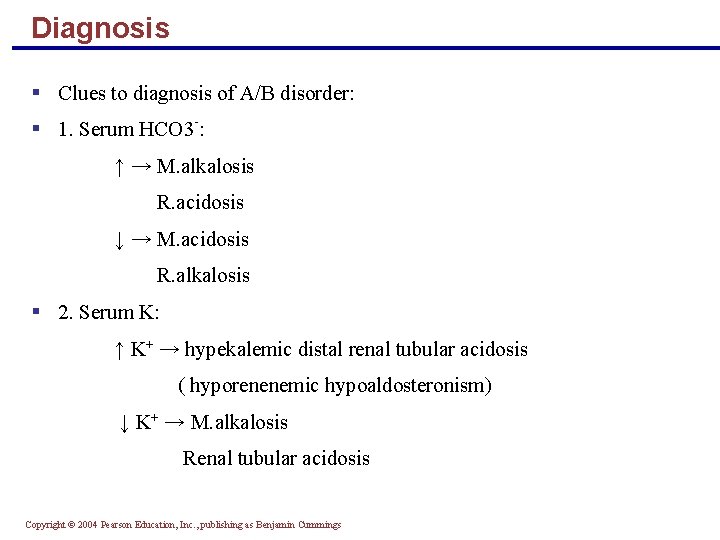
Diagnosis § Clues to diagnosis of A/B disorder: § 1. Serum HCO 3 -: ↑ → M. alkalosis R. acidosis ↓ → M. acidosis R. alkalosis § 2. Serum K: ↑ K+ → hypekalemic distal renal tubular acidosis ( hyporenenemic hypoaldosteronism) ↓ K+ → M. alkalosis Renal tubular acidosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
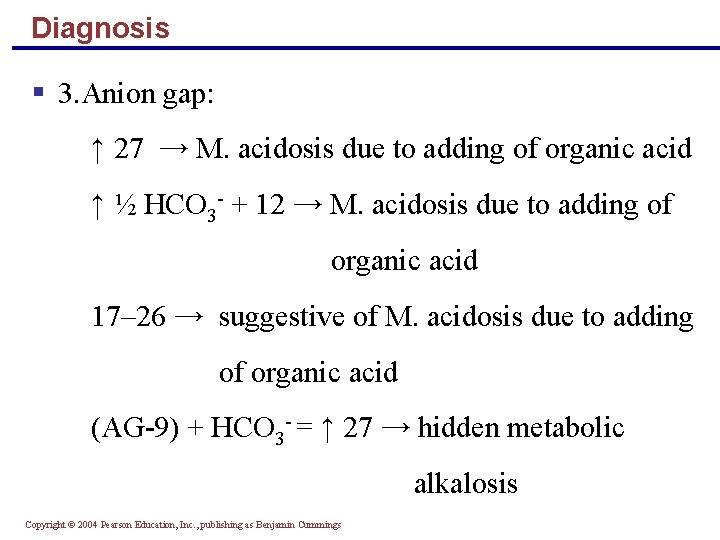
Diagnosis § 3. Anion gap: ↑ 27 → M. acidosis due to adding of organic acid ↑ ½ HCO 3 - + 12 → M. acidosis due to adding of organic acid 17– 26 → suggestive of M. acidosis due to adding of organic acid (AG-9) + HCO 3 - = ↑ 27 → hidden metabolic alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
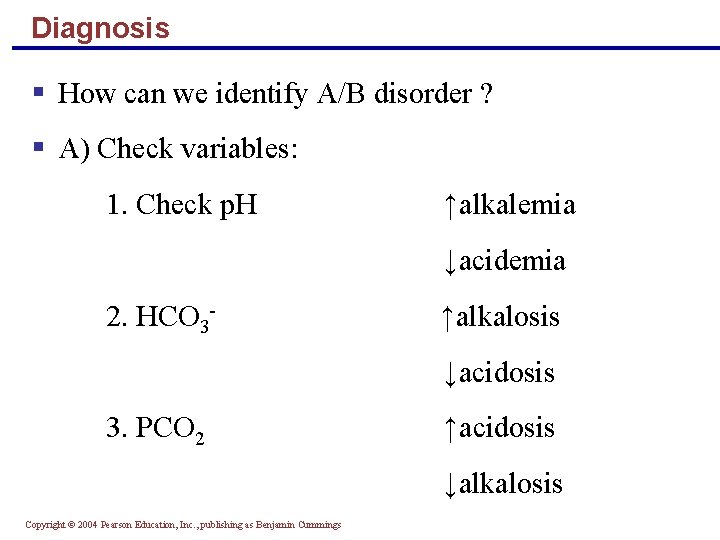
Diagnosis § How can we identify A/B disorder ? § A) Check variables: 1. Check p. H ↑alkalemia ↓acidemia 2. HCO 3 - ↑alkalosis ↓acidosis 3. PCO 2 ↑acidosis ↓alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
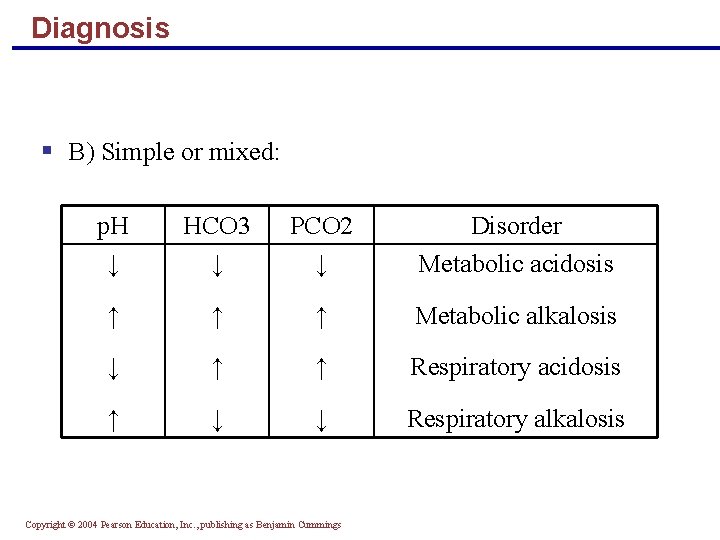
Diagnosis § B) Simple or mixed: p. H ↓ HCO 3 ↓ PCO 2 ↓ Disorder Metabolic acidosis ↑ ↑ ↑ Metabolic alkalosis ↓ ↑ ↑ Respiratory acidosis ↑ ↓ ↓ Respiratory alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
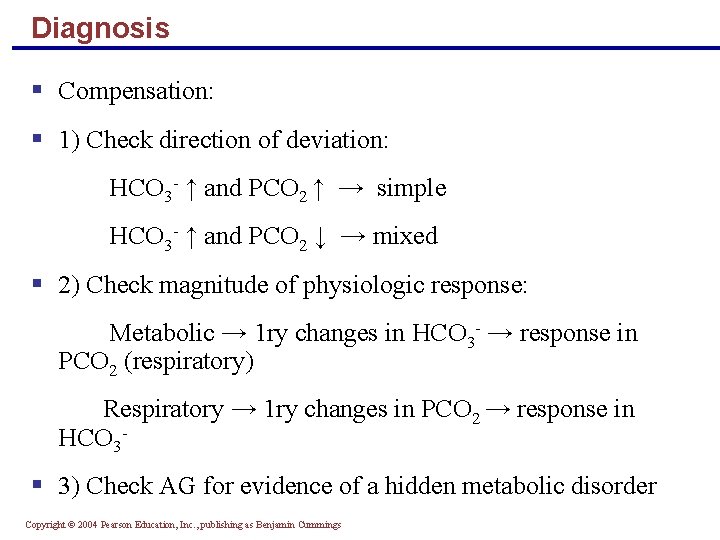
Diagnosis § Compensation: § 1) Check direction of deviation: HCO 3 - ↑ and PCO 2 ↑ → simple HCO 3 - ↑ and PCO 2 ↓ → mixed § 2) Check magnitude of physiologic response: Metabolic → 1 ry changes in HCO 3 - → response in PCO 2 (respiratory) Respiratory → 1 ry changes in PCO 2 → response in HCO 3 - § 3) Check AG for evidence of a hidden metabolic disorder Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
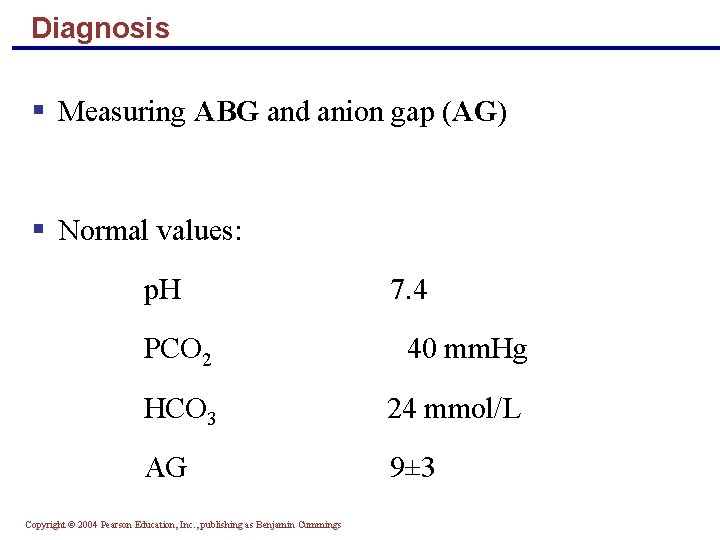
Diagnosis § Measuring ABG and anion gap (AG) § Normal values: p. H 7. 4 PCO 2 40 mm. Hg HCO 3 24 mmol/L AG 9± 3 Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
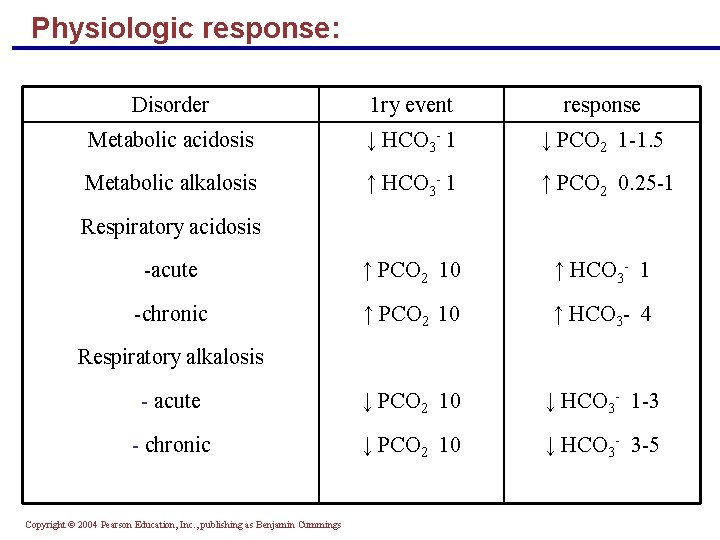
Physiologic response: Disorder 1 ry event response Metabolic acidosis ↓ HCO 3 - 1 ↓ PCO 2 1 -1. 5 Metabolic alkalosis ↑ HCO 3 - 1 ↑ PCO 2 0. 25 -1 -acute ↑ PCO 2 10 ↑ HCO 3 - 1 -chronic ↑ PCO 2 10 ↑ HCO 3 - 4 - acute ↓ PCO 2 10 ↓ HCO 3 - 1 -3 - chronic ↓ PCO 2 10 ↓ HCO 3 - 3 -5 Respiratory acidosis Respiratory alkalosis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
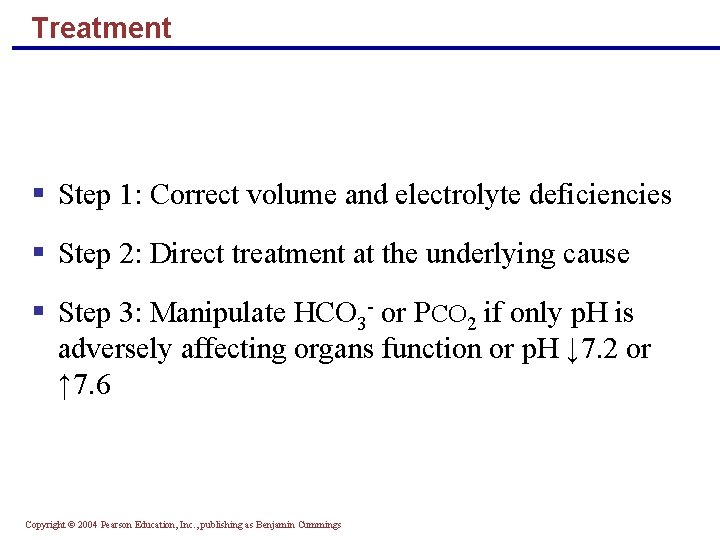
Treatment § Step 1: Correct volume and electrolyte deficiencies § Step 2: Direct treatment at the underlying cause § Step 3: Manipulate HCO 3 - or PCO 2 if only p. H is adversely affecting organs function or p. H ↓ 7. 2 or ↑ 7. 6 Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
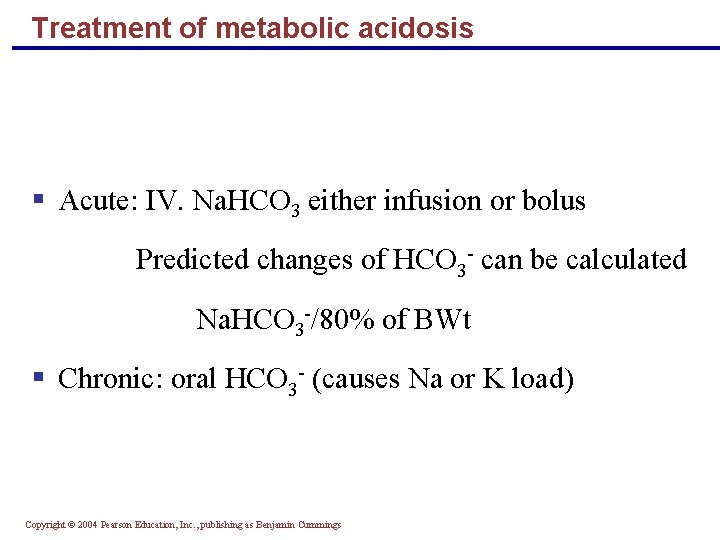
Treatment of metabolic acidosis § Acute: IV. Na. HCO 3 either infusion or bolus Predicted changes of HCO 3 - can be calculated Na. HCO 3 -/80% of BWt § Chronic: oral HCO 3 - (causes Na or K load) Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
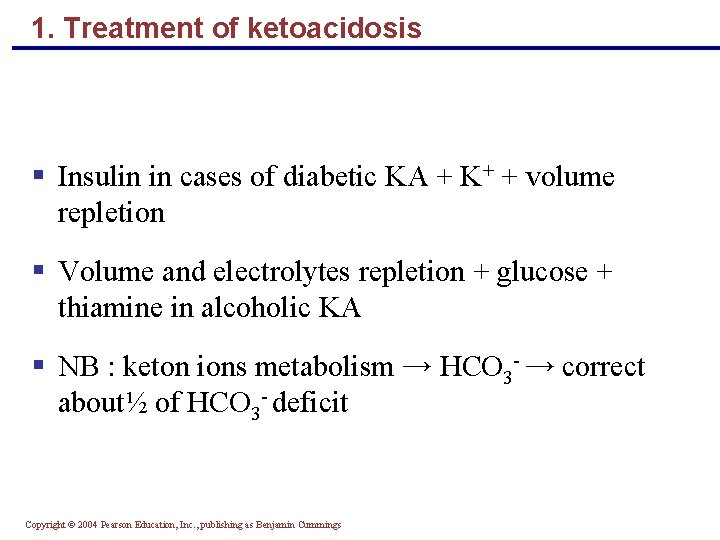
1. Treatment of ketoacidosis § Insulin in cases of diabetic KA + K+ + volume repletion § Volume and electrolytes repletion + glucose + thiamine in alcoholic KA § NB : keton ions metabolism → HCO 3 - → correct about½ of HCO 3 - deficit Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
2. Treatment of lactic acidosis § Restoration of tissue perfusion and O 2 lack § Bolus Na. HCO 3, then § Na. HCO 3 hemodialysis Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

3. Treatment of intoxication § Methanol and ethylene glycol: Immediate ethanol infusion to retard metabolism of alcohol Loading dose 0. 6 -1 g/kg Maintenance dose 10 -20 g/H Immediate dialysis to remove toxins (glycolic acid, formic acid, oxalic acid) Alkalinysation of urine § Salcylates: Alkalinyse the urine by 5% dextrose + 150 mmol HCO 3 -/L Hemodialysis to remove salicylates in - RF - ↓ Mental status - ↑Acidosis - ↓General condition Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Treatment of metabolic alkalosis § 1. Treatment of volume depleted: - ↑↑ Na or K Cl - H 2 blockers (↓ acid loss) - K-sparing diuretics § 2. Treatment of volume replete: - Treat hypermineralocorticoidism - K-sparing diuretics up to 400 mg/d + KCL - Indomethacine in Bartter`s syndrome Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Treatment of metabolic alkalosis cont. § A) In renal failure HD with low HCO 3§ B) Na. Cl If heart failure prevents use of Na. Cl give HCl 150 mmol/L by infusion § NB: - HCl can cause hemolysis and vascular necrosis - NH 4 Cl can be given instead of HCl but it causes gastric distress and ammonium intoxication § c) Acetazolamide: 500 mg/iv in Na. Cl intolerant pts, K+ should be given Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
Treatment of respiratory acidosis § Acid-base disorder in respiratory acidosis does not require treatment because PCO 2 retention even if more than 100 mm. Hg, renal HCO 3 - generation keeps p. H ↑ 7. 2 § Treatment is to ensure adequate O 2 supply Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Treatment of respiratory alkalosis § Correction of the causative disorder is the key § Provision of O 2 is essential Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
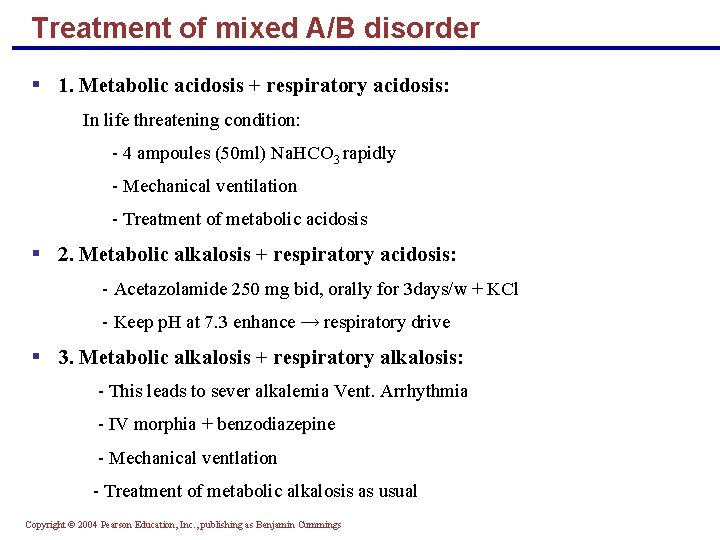
Treatment of mixed A/B disorder § 1. Metabolic acidosis + respiratory acidosis: In life threatening condition: - 4 ampoules (50 ml) Na. HCO 3 rapidly - Mechanical ventilation - Treatment of metabolic acidosis § 2. Metabolic alkalosis + respiratory acidosis: - Acetazolamide 250 mg bid, orally for 3 days/w + KCl - Keep p. H at 7. 3 enhance → respiratory drive § 3. Metabolic alkalosis + respiratory alkalosis: - This leads to sever alkalemia Vent. Arrhythmia - IV morphia + benzodiazepine - Mechanical ventlation - Treatment of metabolic alkalosis as usual Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings

Copyright © 2004 Pearson Education, Inc. , publishing as Benjamin Cummings
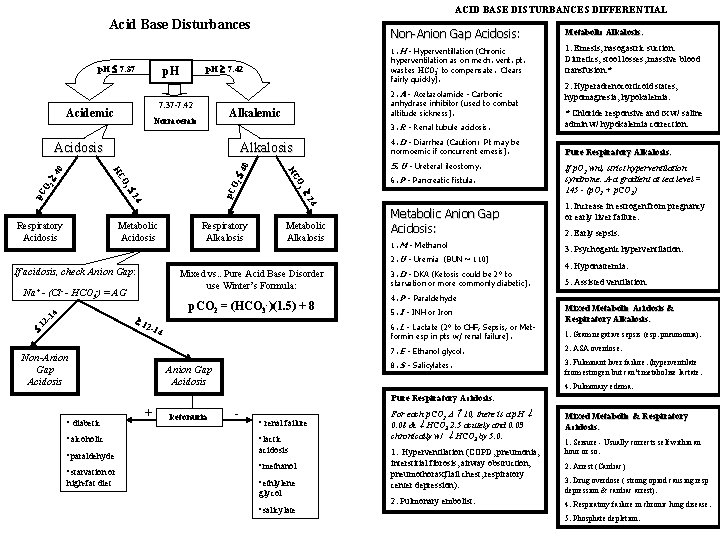
ACID BASE DISTURBANCES DIFFERENTIAL Acid Base Disturbances p. H 7. 42 p. H 7. 37 -7. 42 Acidemic Alkalosis 4. D - Diarrhea (Caution: Pt may be normoemic if concurrent emesis). Pure Respiratory Alkalosis: 5. U - Ureteral ileostomy. 4 4 0 2 O 2 p. C O 4 4 2 2 p. C 6. P - Pancreatic fistula. Respiratory Alkalosis Metabolic Anion Gap Acidosis: 1. M - Methanol 2. U - Uremia (BUN ~ 110) If acidosis, check Anion Gap: Mixed vs. . Pure Acid Base Disorder use Winter’s Formula: 4 Na+ - (Cl- - HCO 3 -) = AG p. CO 2 = (HCO 3 -)(1. 5) + 8 -1 1 12 2 -1 Non-Anion Gap Acidosis 2. Hyperadrenocorticoid states, hypomagnesia, hypokalemia. * Chloride responsive and tx w/ saline admin w/ hypokalemia correction. O 3 HC Metabolic Acidosis 1. Emesis, nasogastric suction. Diuretics, stool losses, massive blood transfusion. * 3. R - Renal tubule acidosis. Acidosis Respiratory Acidosis 1. H - Hyperventillation (Chronic hyperventilation as on mech. vent. pt. wastes HCO 3 - to compensate. Clears fairly quickly). 2. A - Acetazolamide - Carbonic anhydrase inhibitor (used to combat altitude sickness). Alkalemic Normoemic Metabolic Alkalosis: 0 p. H 7. 37 Non-Anion Gap Acidosis: Acidosis 3. D - DKA (Ketosis could be 2° to starvation or more commonly diabetic). Anion Gap Acidosis 1. Increase in estrogen from pregnancy or early liver failure. 2. Early sepsis. 3. Psychogenic hyperventilation. 4. Hyponatremia. 5. Assisted ventilation. 4. P - Paraldehyde 5. I - INH or Iron 6. L - Lactate (2° to CHF, Sepsis, or Metformin esp in pts w/ renal failure). 4 If p. O 2 wnl, strict hyperventilation syndrome. A-a gradient at sea level = 145 - (p. O 2 + p. CO 2) Mixed Metabolic Acidosis & Respiratory Alkalosis: 1. Gram negative sepsis (esp. pneumonia). 7. E - Ethanol glycol. 2. ASA overdose. 8. S - Salicylates. 3. Fulminant liver failure. (hyperventilate from estrogen but can’t metabolize lactate. 4. Pulmonary edema. Pure Respiratory Acidosis: • diabetic • alcoholic • paraldehyde • starvation or high-fat diet + ketonuria - • renal failure • lactic acidosis • methanol • ethlylene glycol • salicylate For each p. CO 2 10, there is a p. H 0. 08 & HCO 3 2. 5 acutely and 0. 03 chronically w/ HCO 3 by 5. 0. Mixed Metabolic & Respiratory Acidosis: 1. Hyperventilation (COPD, pneumonia, interstitial fibrosis, airway obstruction, pneumothorax, flail chest, respiratory center depression). 2. Arrest (Cardiac) 2. Pulmonary embolist. 1. Seizure - Usually corrects self within an hour or so. 3. Drug overdose ( strong opiod causing resp depression & cardiac arrest). 4. Respiratory failure in chronic lung disease. 5. Phosphate depletion.
- Slides: 79