Copper vinegar peroxide salt Isopropyl Alcohol Lab Demo

Copper, vinegar, peroxide, salt Isopropyl Alcohol Lab Demo BLUE LAB Literacy: 1. Do we grow it or mine it 2. The secrets of rust COMBUSTION OF ISOPROPYL ALCOHOL LAB DEMO NOTE BOOK PAGE 30 Balancing Chemical Equations
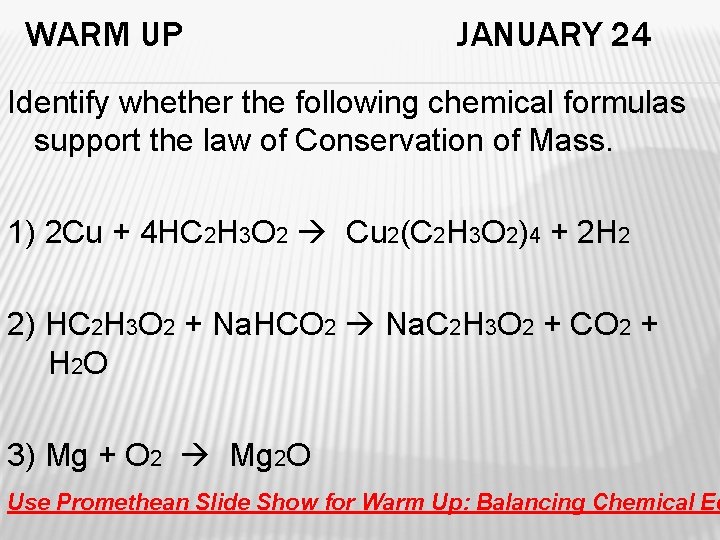
WARM UP JANUARY 24 Identify whether the following chemical formulas support the law of Conservation of Mass. 1) 2 Cu + 4 HC 2 H 3 O 2 Cu 2(C 2 H 3 O 2)4 + 2 H 2 2) HC 2 H 3 O 2 + Na. HCO 2 Na. C 2 H 3 O 2 + CO 2 + H 2 O 3) Mg + O 2 Mg 2 O Use Promethean Slide Show for Warm Up: Balancing Chemical Eq
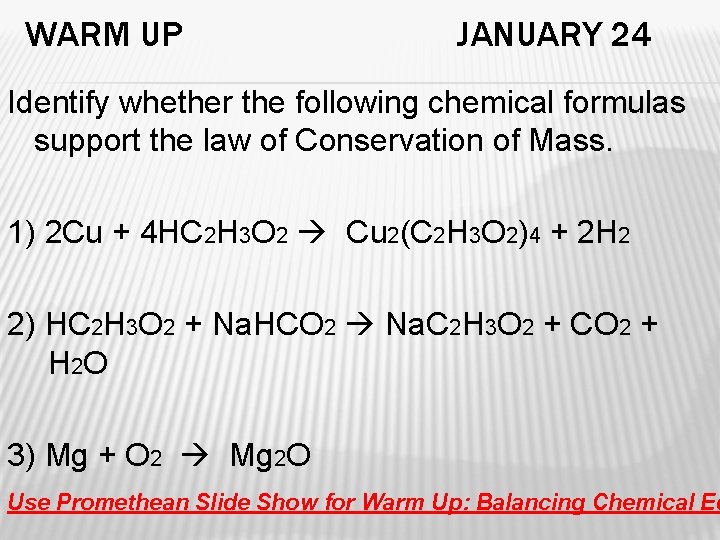
WARM UP JANUARY 24 Identify whether the following chemical formulas support the law of Conservation of Mass. 1) 2 Cu + 4 HC 2 H 3 O 2 Cu 2(C 2 H 3 O 2)4 + 2 H 2 2) HC 2 H 3 O 2 + Na. HCO 2 Na. C 2 H 3 O 2 + CO 2 + H 2 O 3) Mg + O 2 Mg 2 O Use Promethean Slide Show for Warm Up: Balancing Chemical Eq

WARM UP JANUARY 22, 2020 1. Balance the following chemical reactions: follow the 6 steps. 1. Cu + O 2 Cu. O C 3 H 8 + O 2 CO 2 + H 2 O 2. Mr. Black mixes 10 grams of baking soda + 10 grams of vinegar. According to the law of conservation of mass, what should be the final weight of the product be after a chemical reaction? Explain why… 3. Spiral: Which of the following would be an example of a pure substance? a) gold b) sugar c) water d)sweet tea 1.
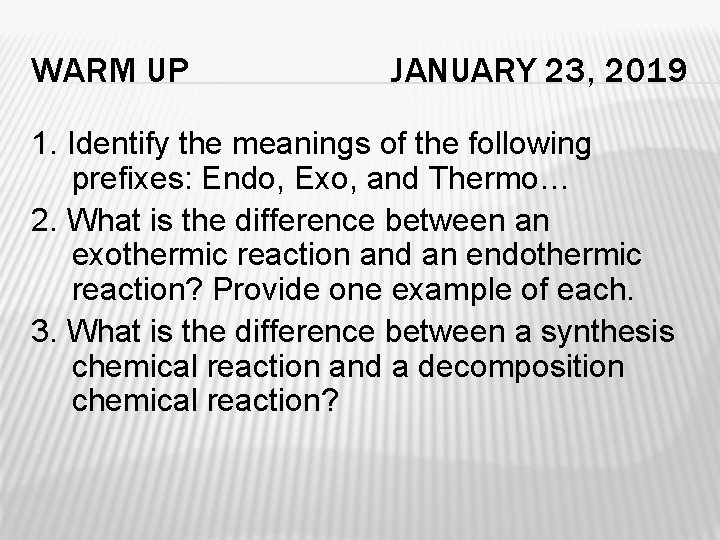
WARM UP JANUARY 23, 2019 1. Identify the meanings of the following prefixes: Endo, Exo, and Thermo… 2. What is the difference between an exothermic reaction and an endothermic reaction? Provide one example of each. 3. What is the difference between a synthesis chemical reaction and a decomposition chemical reaction? 1.

WRITE AND BALANCE… THE COMBUSTION OF ISOPROPYL ALCOHOL LAB DEMO 1/24/19 Warm Up 1/23/20 New Quarter 3 T. O. C. and Warm Up Page 1 1. Balance the following chemical reactions: Ag 2 O Ag + O 2 / P + O 2 P 2 O 5 2. How can you determine if a chemical reaction has occurred during an experiment? Today’s Objectives: 8. P. 1. 3 & 1. 4
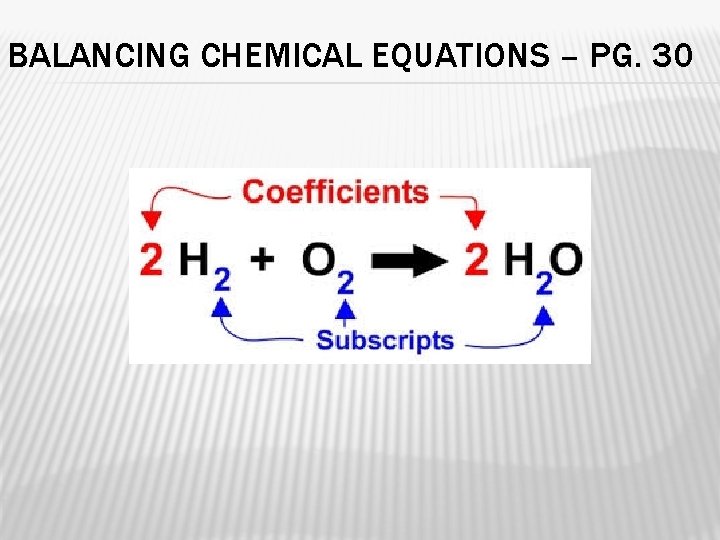
BALANCING CHEMICAL EQUATIONS – PG. 30
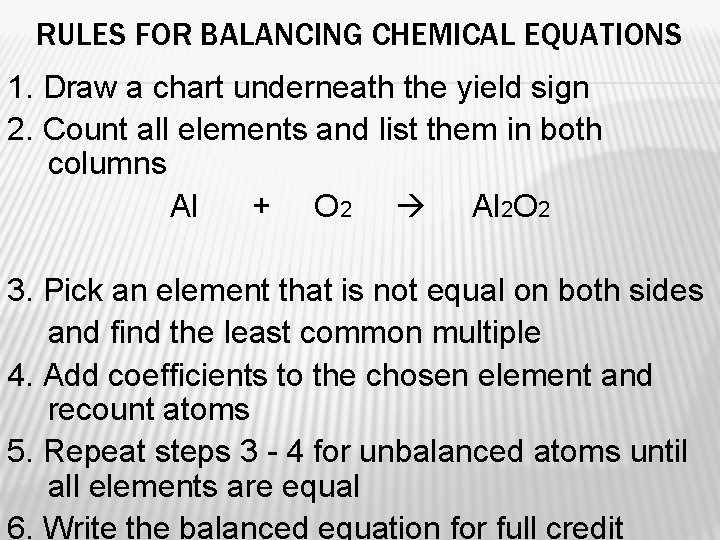
RULES FOR BALANCING CHEMICAL EQUATIONS 1. Draw a chart underneath the yield sign 2. Count all elements and list them in both columns Al + O 2 Al 2 O 2 3. Pick an element that is not equal on both sides and find the least common multiple 4. Add coefficients to the chosen element and recount atoms 5. Repeat steps 3 - 4 for unbalanced atoms until all elements are equal 6. Write the balanced equation for full credit
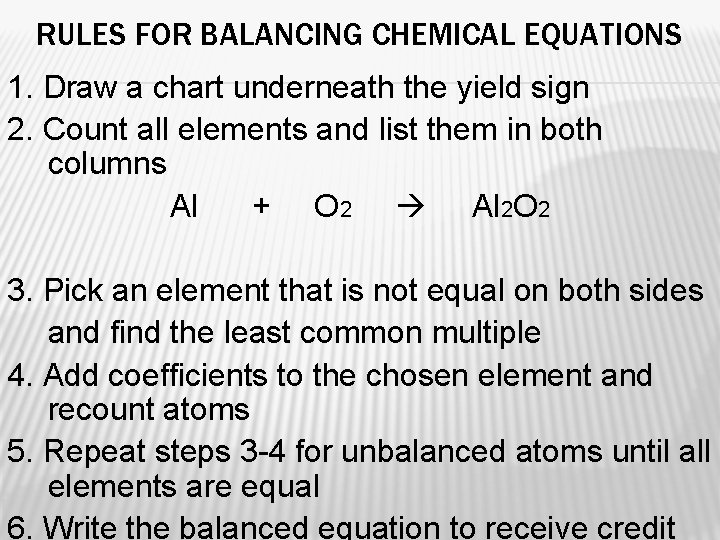
RULES FOR BALANCING CHEMICAL EQUATIONS 1. Draw a chart underneath the yield sign 2. Count all elements and list them in both columns Al + O 2 Al 2 O 2 3. Pick an element that is not equal on both sides and find the least common multiple 4. Add coefficients to the chosen element and recount atoms 5. Repeat steps 3 -4 for unbalanced atoms until all elements are equal 6. Write the balanced equation to receive credit
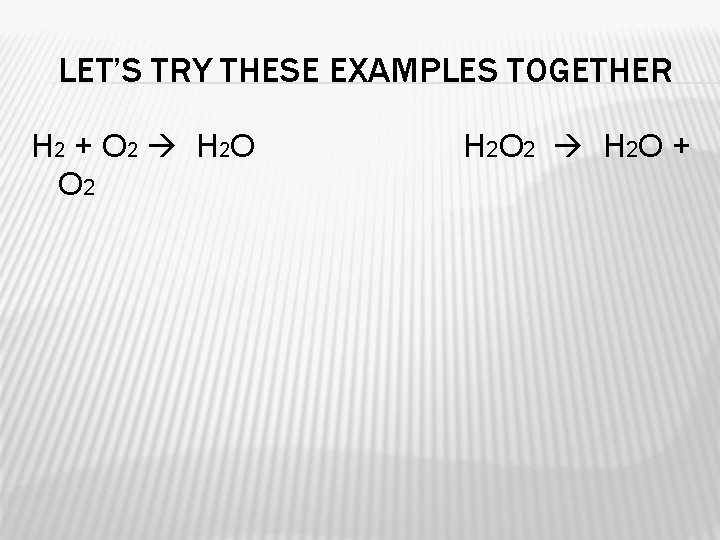
LET’S TRY THESE EXAMPLES TOGETHER H 2 + O 2 H 2 O O 2 H 2 O 2 H 2 O +
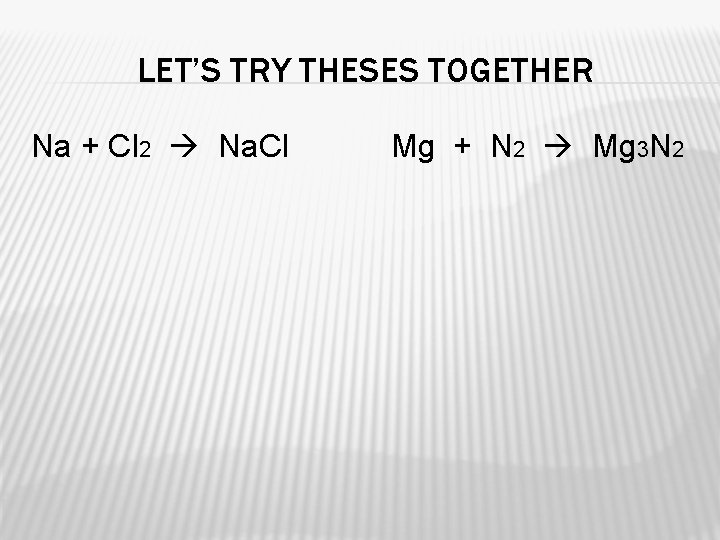
LET’S TRY THESES TOGETHER Na + Cl 2 Na. Cl Mg + N 2 Mg 3 N 2
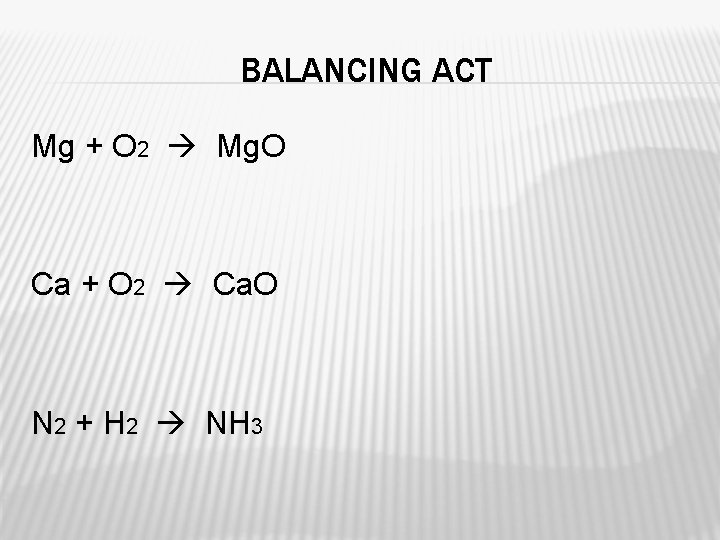
BALANCING ACT Mg + O 2 Mg. O Ca + O 2 Ca. O N 2 + H 2 NH 3
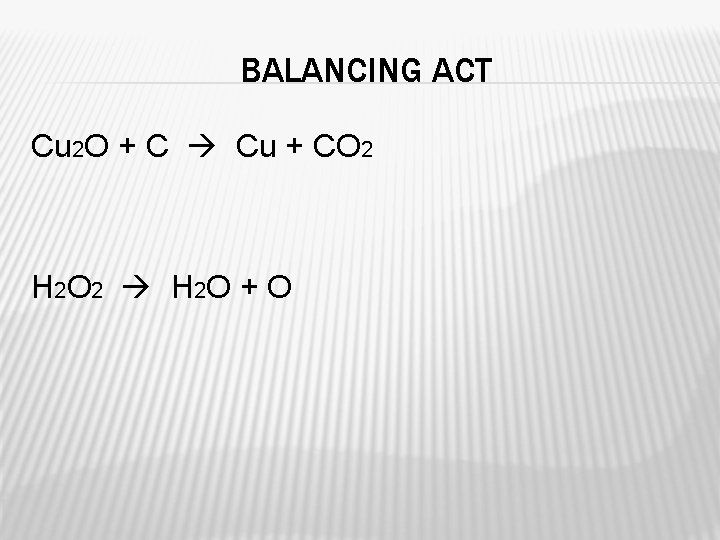
BALANCING ACT Cu 2 O + C Cu + CO 2 H 2 O 2 H 2 O + O
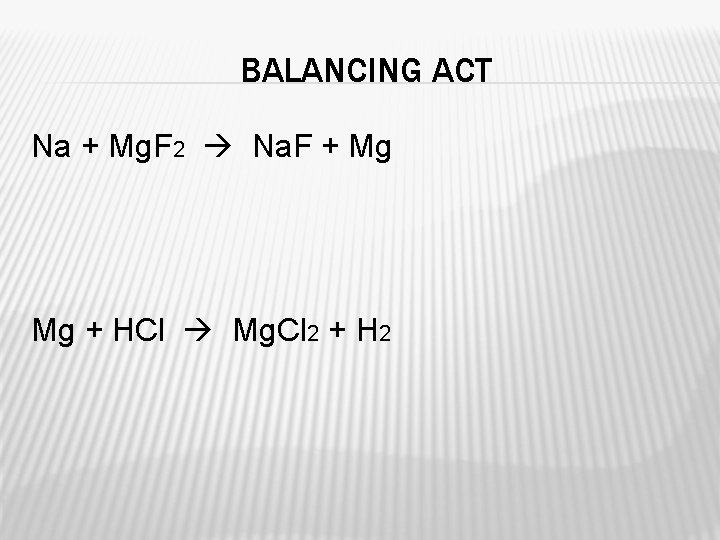
BALANCING ACT Na + Mg. F 2 Na. F + Mg Mg + HCl Mg. Cl 2 + H 2
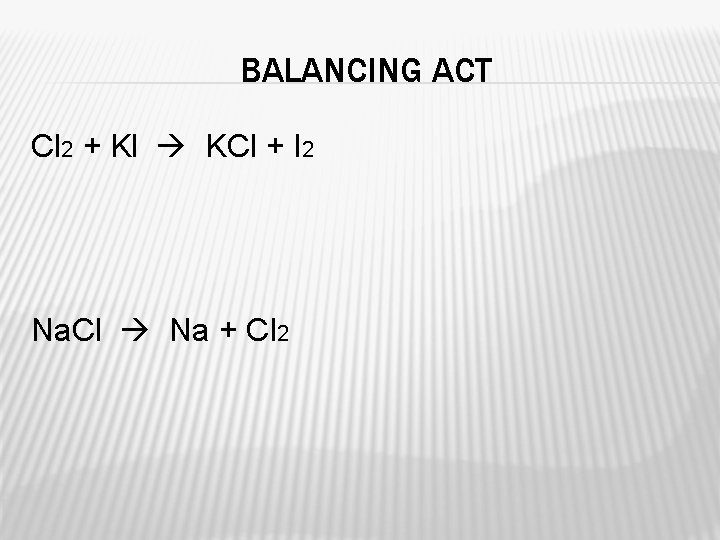
BALANCING ACT Cl 2 + Kl KCl + I 2 Na. Cl Na + Cl 2


2 Na. Cl + F 2 2 Na. F + Cl 2 3 Fe + 4 H 2 O Fe 3 O 4 +4 H 2

COMBUSTION OF ISOPROPYL ALCOHOL Hypothesis: What will happen during a combustion chemical reaction? _________________________________________________

COMBUSTION OF ISOPROPYL ALCOHOL
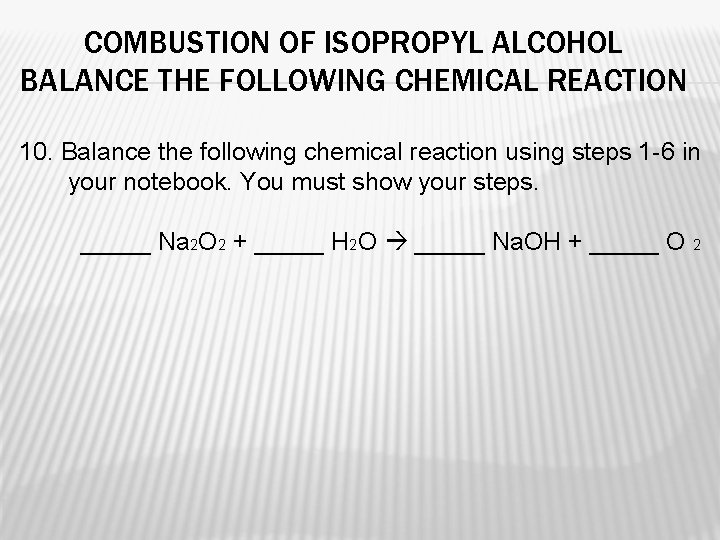
COMBUSTION OF ISOPROPYL ALCOHOL BALANCE THE FOLLOWING CHEMICAL REACTION 10. Balance the following chemical reaction using steps 1 -6 in your notebook. You must show your steps. _____ Na 2 O 2 + _____ H 2 O _____ Na. OH + _____ O 2

DISCUSSION QUESTIONS: 1. CAN YOU TELL WHETHER ENERGY WAS ABSORBED OR RELEASED IN THIS REACTION? EXPLAIN HOW YOU KNOW… 2. DOES THAT MAKE THIS REACTION ENDOTHERMIC OR EXOTHERMIC? EXPLAIN… 3. WHICH SIDE WOULD ENERGY BE FOUND ON? Energy is released with this reaction: We call this EXOTHERMIC for “EXIT HEAT” 2 C 3 H 8 O + 9 O 2 6 CO 2 + 8 H 2 O+ energy




INTRODUCTION TO ENERGY RESOURCES 1. Read the article: “Do We Grow It or Mine It? ” 2. As a group, Go back through the article and complete the comparing and contrasting energy resources worksheet. 3. Review and discuss answers as a group. 4. Review as a class.
- Slides: 25