Copper Transporters CTR 1 CTR 2 ATP 7



- Slides: 17

Copper Transporters: CTR 1, CTR 2, ATP 7 A, & ATP 7 B Veronica Lopez Stefano Pineda Jude Okolo

Copper and Copper Transporters � Useful because of its ability to reduce to Copper( I) from Copper(II) � Functions in processes such as mitochondrial respiration, iron transport, hormone production, neurotransmitter synthesis, and pigmentation. � Copper transporters maintain homeostasis of copper levels at the cellular level � Many copper transporters focus here on CTR 1 CTR 5, ATP 7 A, and ATP 7 B

Menkes Syndrome and Wilson Disease � Menkes syndrome X-linked recessive disorder defective ATP 7 A – ◦ Characterized by copper deficiency ◦ Menkes affects delivery of copper to the brain and incorporation of copper in different enzymes ◦ Symptoms include decreased neural development, seizures, and connective tissue abnormalities � Wilson Disease autosomal recessive disease causes defect in ATP 7 B ◦ Characterized by copper overload ◦ Wilson causes toxic accumulation of copper in the brain by decreased copper excretion by the liver into the bile ◦ Symptoms include hepatic abnormalities leading to liver failure, neurological defects, and psychiatric symptoms

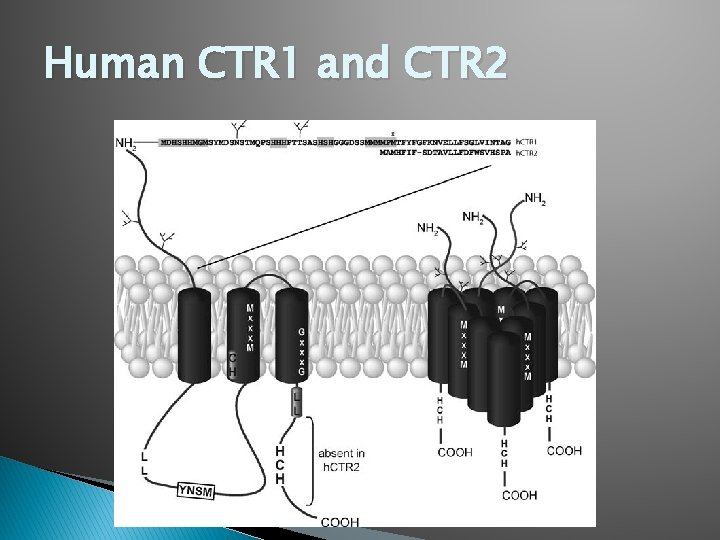
Human CTR 1 and CTR 2

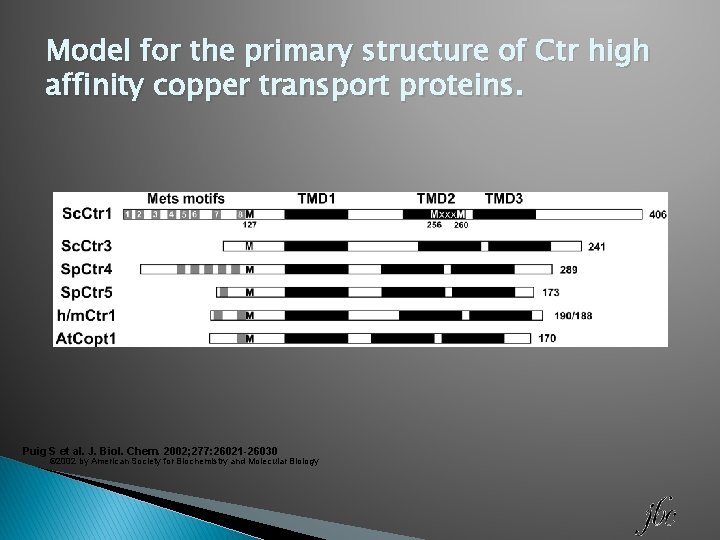
CTR 1 and CTR 2: Primary Structure � YCTR 1 and CTR 2 have 25% homology and are most similar in the three transmembrane regions � CTR 1 has an amino terminal region rich in Met and His and an MXXXM motif in TM 2 � Both CTR 1 and CTR 2 have a Met 20 AAs upstream from TM 1 � CTR 1 made up of 190 amino acids CTR 2 is made up of slightly less.

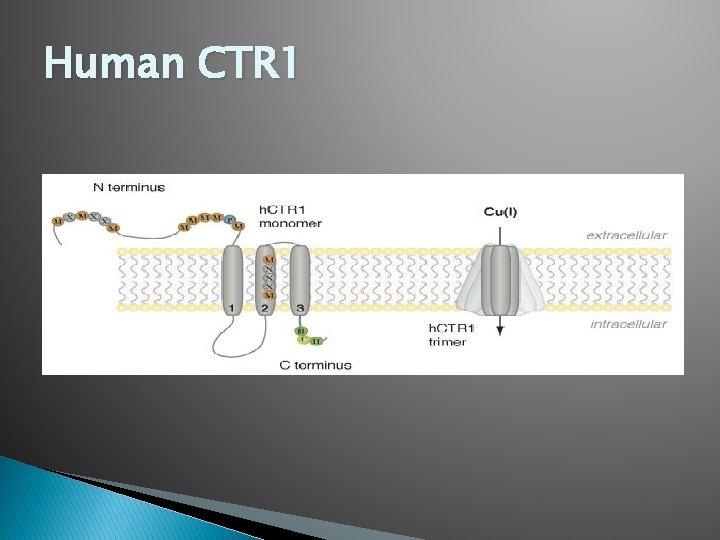
CTR 1 and CTR 2: Secondary Structure � CTR proteins in general have three transmembrane α-helices. � N-terminus is located in the extracellular space � C-terminus is located in intracellular space � CTR 2 lacks the extended N-terminus of CTR 1 and the Met motifs may be the cause of decreased affinity for copper transport � GG 4 motif has role in helix-helix interactions in other proteins.

Model for the primary structure of Ctr high affinity copper transport proteins. Puig S et al. J. Biol. Chem. 2002; 277: 26021 -26030 © 2002 by American Society for Biochemistry and Molecular Biology

CTR 1: Tertiary and Quaternary Structure � CTR 1 proteins monomers consist of three transmembrane domains (TMD) � Each monomer makes up a homotrimer to form a copper transport core. � TMD 2 and TMD 3 have MXXXM and GXXXG motifs � The Met motif in TMD 2 may play a role in copper sensing and uptake. � GG 4 motif essential to formation of functional copper uptake proteins.

Human CTR 1

CTR 2: Tertiary and Quaternary Structure � CTR 2 protein has three transmembrane domains � Monomers exist as homomultimers � Much less in known about CTR 2

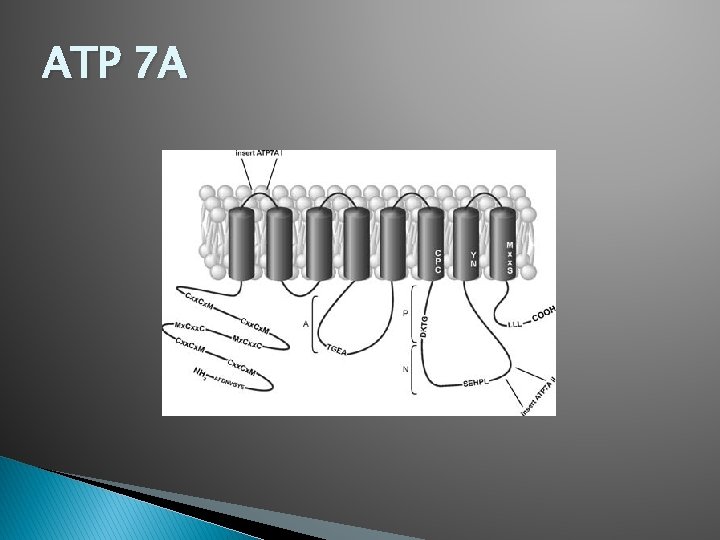
ATP 7 A

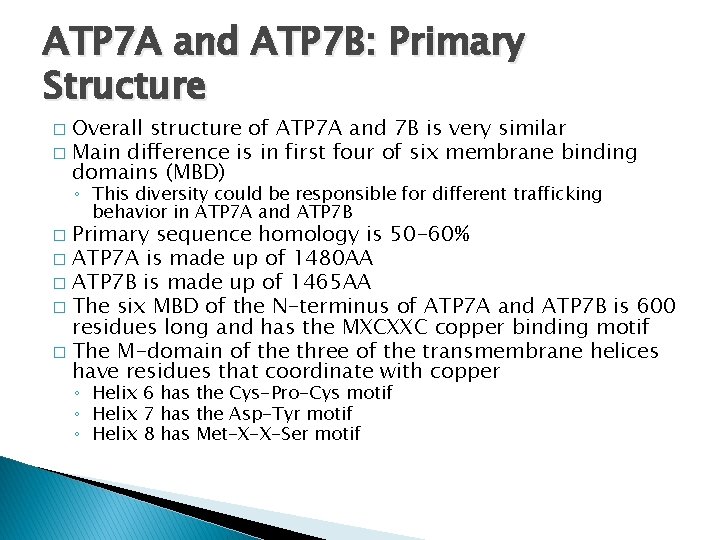
ATP 7 A and ATP 7 B: Primary Structure Overall structure of ATP 7 A and 7 B is very similar � Main difference is in first four of six membrane binding domains (MBD) � ◦ This diversity could be responsible for different trafficking behavior in ATP 7 A and ATP 7 B Primary sequence homology is 50 -60% � ATP 7 A is made up of 1480 AA � ATP 7 B is made up of 1465 AA � The six MBD of the N-terminus of ATP 7 A and ATP 7 B is 600 residues long and has the MXCXXC copper binding motif � The M-domain of the three of the transmembrane helices have residues that coordinate with copper � ◦ Helix 6 has the Cys-Pro-Cys motif ◦ Helix 7 has the Asp-Tyr motif ◦ Helix 8 has Met-X-X-Ser motif

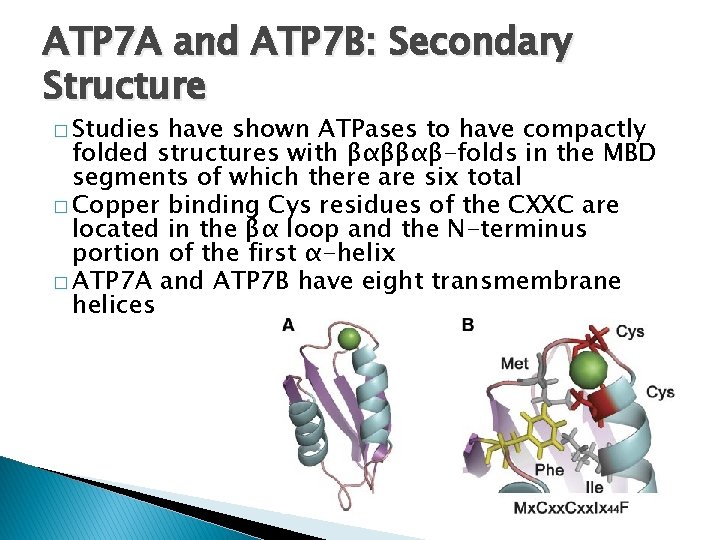
ATP 7 A and ATP 7 B: Secondary Structure � Studies have shown ATPases to have compactly folded structures with βαββαβ-folds in the MBD segments of which there are six total � Copper binding Cys residues of the CXXC are located in the βα loop and the N-terminus portion of the first α-helix � ATP 7 A and ATP 7 B have eight transmembrane helices

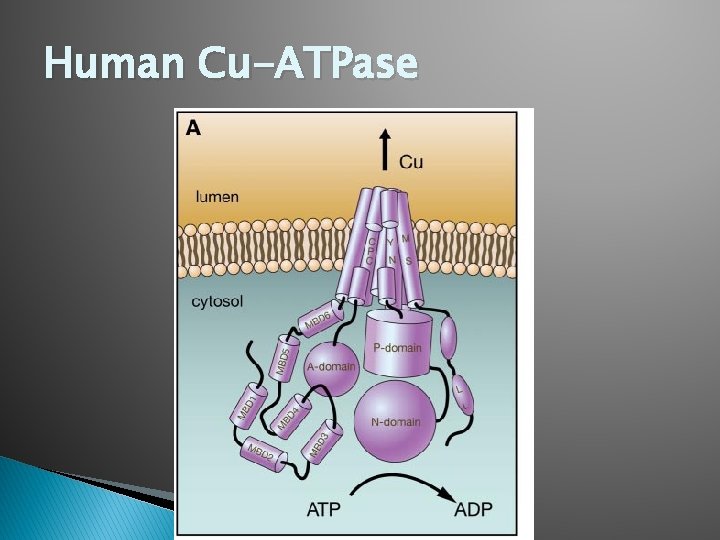
ATP 7 A and ATP 7 B: Tertiary Structure � MBD 1 -4 facilitate binding and hydrolysis of ATP � MBD 5 and 6 are necessary for copper binding in the transmembrane portion of transporter � There are eight TMS in the transmembrane portion of Cu-ATPases � A CPC motif in the TMS is highly conserved along with four other AA in segments 7 and 8 and are likely to form copper binding sites within the membrane. � The A-Domain, P-Domain, and N-Domain all function in ATP binding and catalytic phosphorylation of ATP

Human Cu-ATPase

Comparing Copper Transport Proteins � Much less is known about the structure of CTR 1 and CTR 2 and much more is known about ATP 7 A and ATP 7 B ◦ ATP 7 A and ATP 7 B are much more similar to one another than CTR 1 and CTR 2 and ATP 7 A and B are part of large family of P-type ATPases � CTR 1 and 2 function in copper import at cell membrane while ATP 7 A and ATP 7 B function in copper transport into trans Golgi network and export of Copper at the

References � Van den Burghe, Peter V. E. and Klomp, Leo W. J. Posttranslational Regulation of Copper Transporters. Journal of Biological Inorganic Chemistry (2010) 15: 37 -46 � Lutsenko, Svetlana et. al. Function and Regulation of Copper-Transporting ATPases. The American Physiological Society (2007) 87: 1011 -1046 � Puig, Sergi, et. al. Biochemical and Genetic Analyses of Yeast and Human High Affinity Copper Transporters Suggest a Conserved Mechanism for Copper Uptake The Journal of Biological Chemistry (2002) 29: 26021 -26030 � De Bie, P. , Muller, P. , et. al. Molecular Pathogenesis of Wilson and Menkes Disease: Correlation of Mutations with Molecular Effects and Disease Phenotypes. Journal of Medical Genetics (2007) 44: 673 -688