COPD clinical update latest evidence and guidelines Dr












- Slides: 65

COPD clinical update: latest evidence and guidelines Dr Richa Singh May 2018

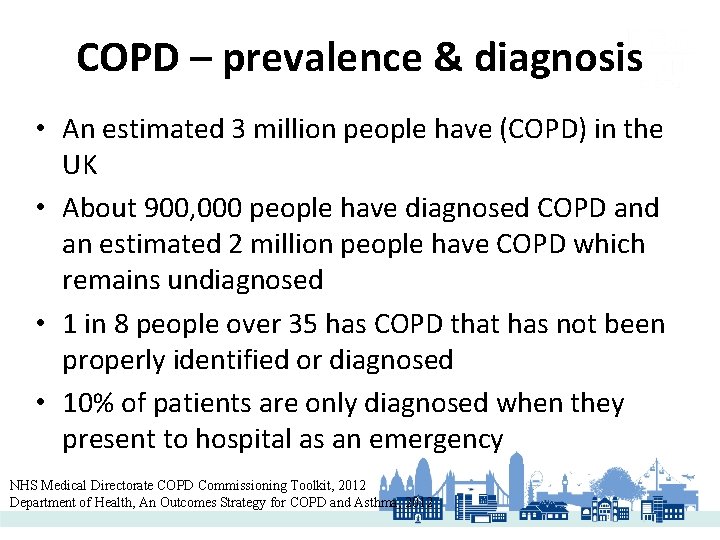
COPD – prevalence & diagnosis • An estimated 3 million people have (COPD) in the UK • About 900, 000 people have diagnosed COPD and an estimated 2 million people have COPD which remains undiagnosed • 1 in 8 people over 35 has COPD that has not been properly identified or diagnosed • 10% of patients are only diagnosed when they present to hospital as an emergency NHS Medical Directorate COPD Commissioning Toolkit, 2012 Department of Health, An Outcomes Strategy for COPD and Asthma, 2012

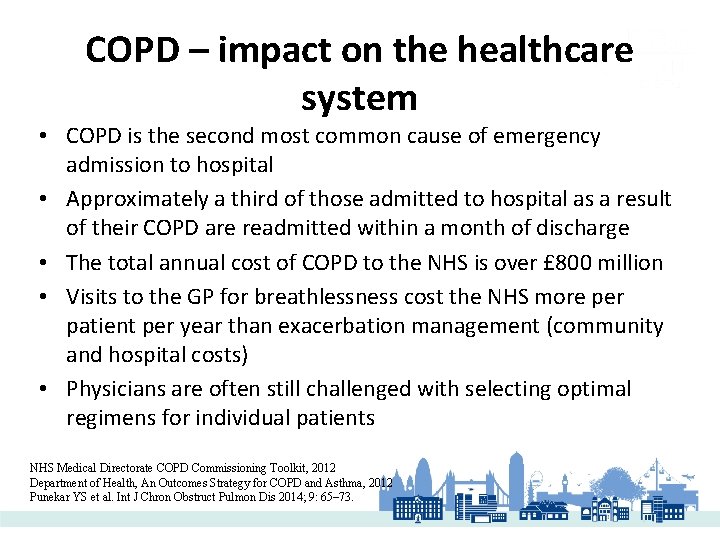
COPD – impact on the healthcare system • COPD is the second most common cause of emergency admission to hospital • Approximately a third of those admitted to hospital as a result of their COPD are readmitted within a month of discharge • The total annual cost of COPD to the NHS is over £ 800 million • Visits to the GP for breathlessness cost the NHS more per patient per year than exacerbation management (community and hospital costs) • Physicians are often still challenged with selecting optimal regimens for individual patients NHS Medical Directorate COPD Commissioning Toolkit, 2012 Department of Health, An Outcomes Strategy for COPD and Asthma, 2012 Punekar YS et al. Int J Chron Obstruct Pulmon Dis 2014; 9: 65– 73.

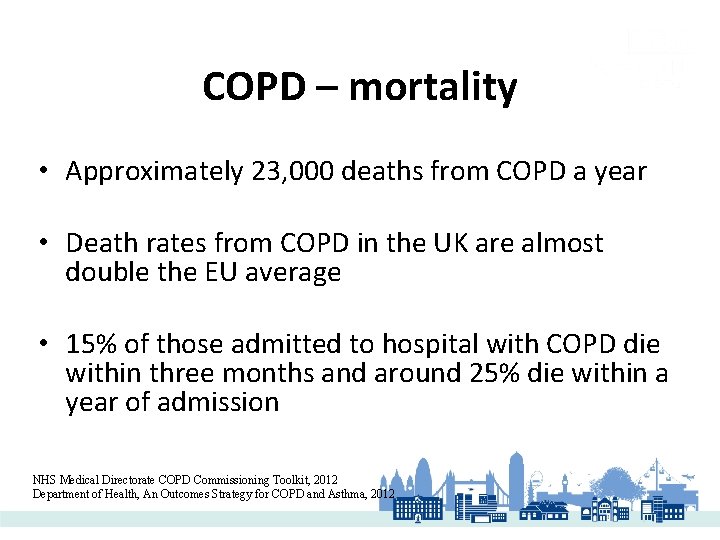
COPD – mortality • Approximately 23, 000 deaths from COPD a year • Death rates from COPD in the UK are almost double the EU average • 15% of those admitted to hospital with COPD die within three months and around 25% die within a year of admission NHS Medical Directorate COPD Commissioning Toolkit, 2012 Department of Health, An Outcomes Strategy for COPD and Asthma, 2012

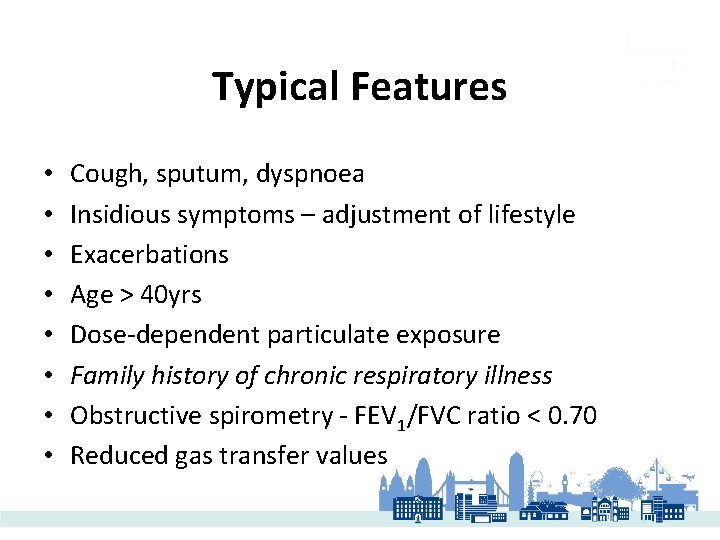
Typical Features • • Cough, sputum, dyspnoea Insidious symptoms – adjustment of lifestyle Exacerbations Age > 40 yrs Dose-dependent particulate exposure Family history of chronic respiratory illness Obstructive spirometry - FEV 1/FVC ratio < 0. 70 Reduced gas transfer values

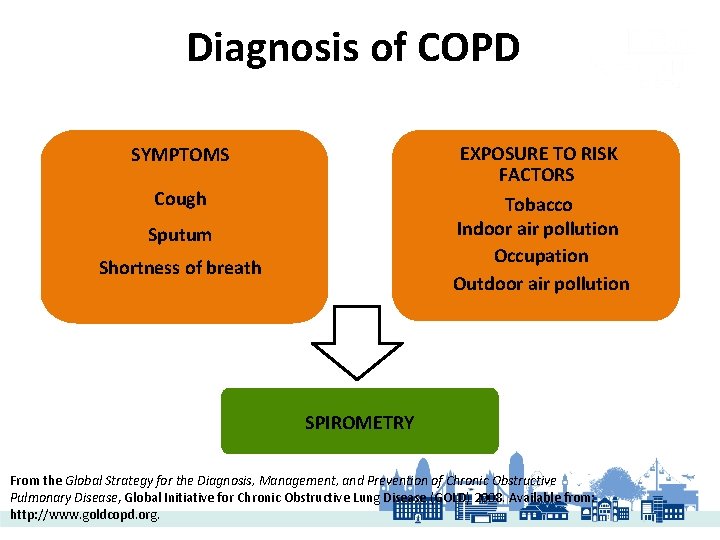
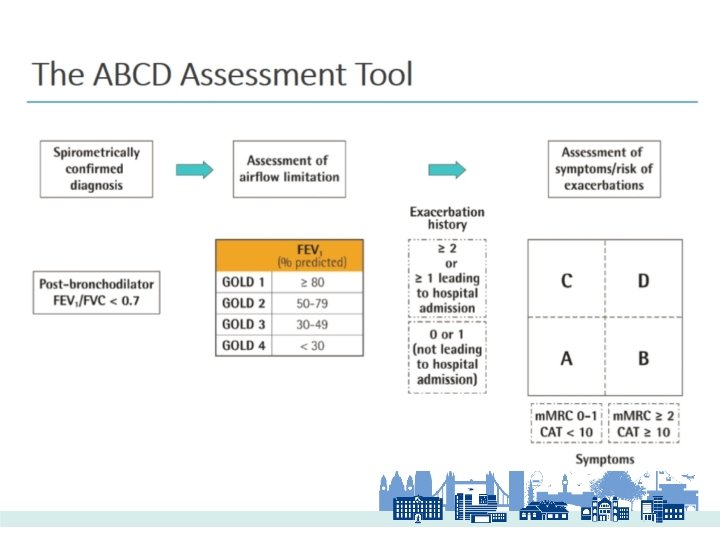
Diagnosis of COPD EXPOSURE TO RISK FACTORS Tobacco Indoor air pollution Occupation Outdoor air pollution SYMPTOMS Cough Sputum Shortness of breath SPIROMETRY From the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2008. Available from: http: //www. goldcopd. org.

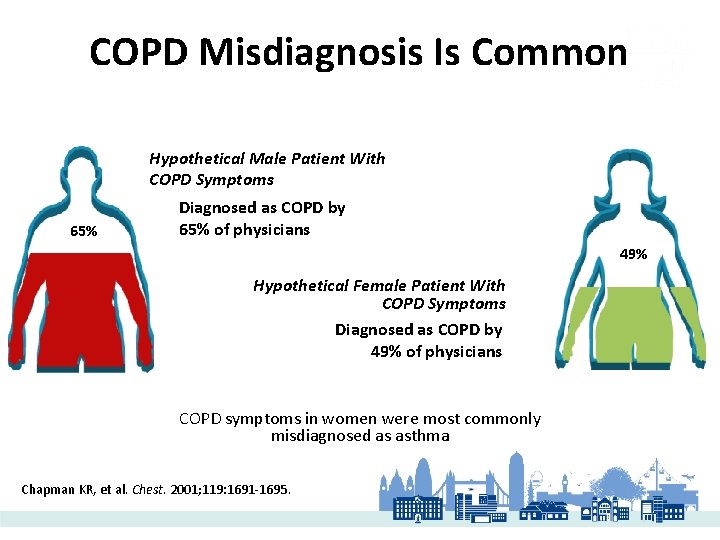
COPD Misdiagnosis Is Common Hypothetical Male Patient With COPD Symptoms 65% Diagnosed as COPD by 65% of physicians 49% Hypothetical Female Patient With COPD Symptoms Diagnosed as COPD by 49% of physicians COPD symptoms in women were most commonly misdiagnosed as asthma Chapman KR, et al. Chest. 2001; 119: 1691 -1695.

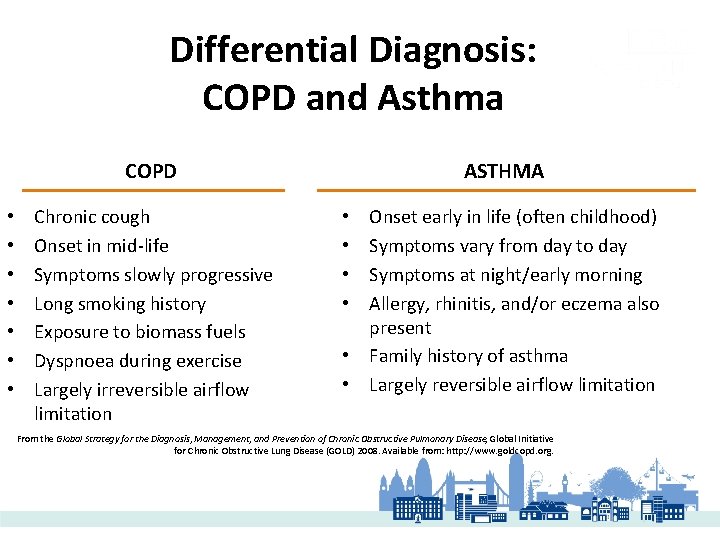
Differential Diagnosis: COPD and Asthma COPD • • Chronic cough Onset in mid-life Symptoms slowly progressive Long smoking history Exposure to biomass fuels Dyspnoea during exercise Largely irreversible airflow limitation ASTHMA Onset early in life (often childhood) Symptoms vary from day to day Symptoms at night/early morning Allergy, rhinitis, and/or eczema also present • Family history of asthma • Largely reversible airflow limitation • • From the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2008. Available from: http: //www. goldcopd. org.

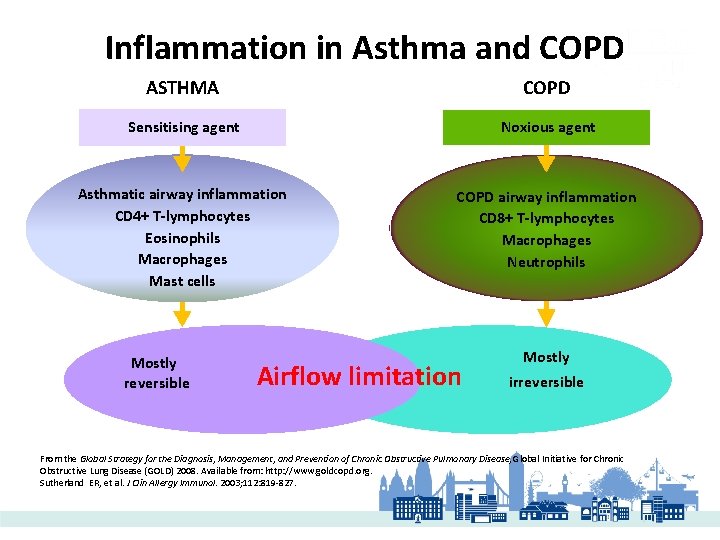
Inflammation in Asthma and COPD ASTHMA COPD Sensitising agent Noxious agent Asthmatic airway inflammation CD 4+ T-lymphocytes Eosinophils Macrophages Mast cells COPD airway inflammation CD 8+ T-lymphocytes Macrophages Neutrophils Mostly reversible Airflow limitation Mostly irreversible From the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2008. Available from: http: //www. goldcopd. org. Sutherland ER, et al. J Clin Allergy Immunol. 2003; 112: 819 -827.

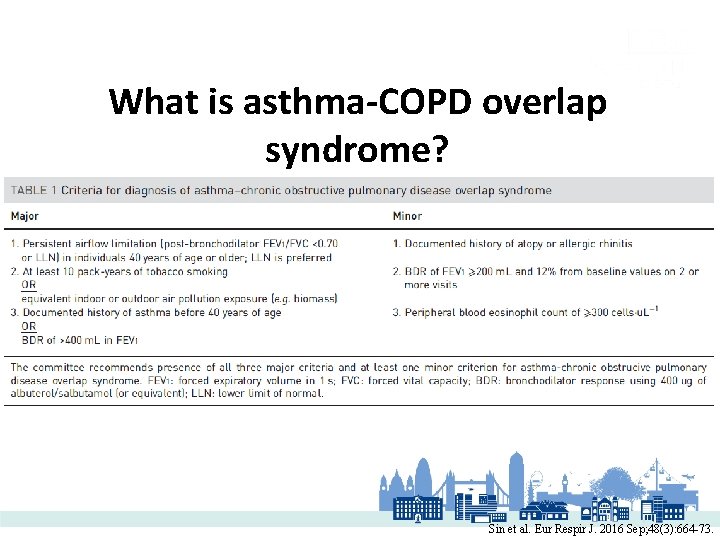
What is asthma-COPD overlap syndrome? Sin et al. Eur Respir J. 2016 Sep; 48(3): 664 -73.

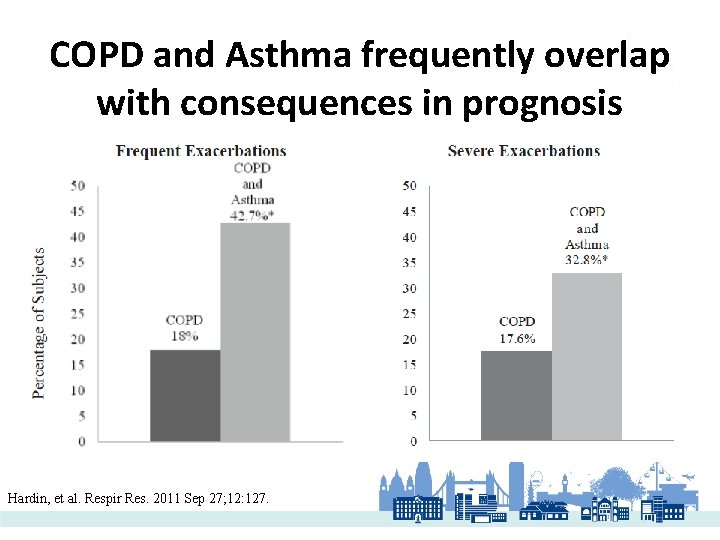
COPD and Asthma frequently overlap with consequences in prognosis Hardin, et al. Respir Res. 2011 Sep 27; 12: 127.

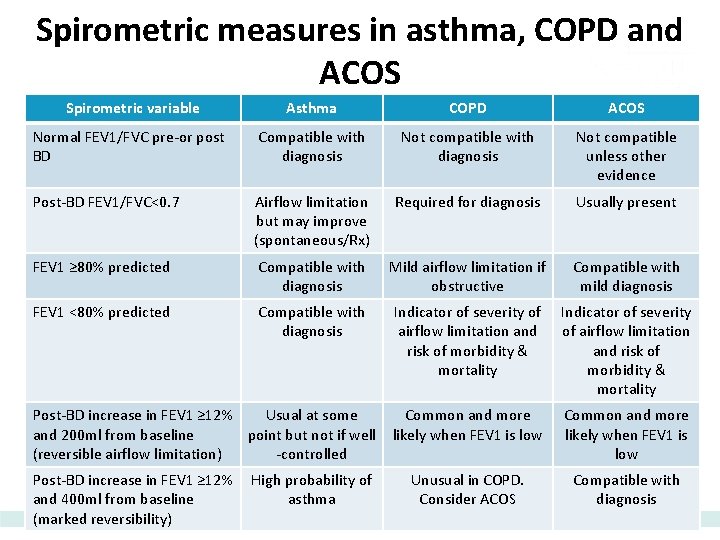
Spirometric measures in asthma, COPD and ACOS Spirometric variable Asthma COPD ACOS Normal FEV 1/FVC pre-or post BD Compatible with diagnosis Not compatible unless other evidence Post-BD FEV 1/FVC<0. 7 Airflow limitation but may improve (spontaneous/Rx) Required for diagnosis Usually present FEV 1 ≥ 80% predicted Compatible with diagnosis Mild airflow limitation if obstructive Compatible with mild diagnosis FEV 1 <80% predicted Compatible with diagnosis Indicator of severity of airflow limitation and risk of morbidity & mortality Common and more likely when FEV 1 is low Unusual in COPD. Consider ACOS Compatible with diagnosis Post-BD increase in FEV 1 ≥ 12% Usual at some and 200 ml from baseline point but not if well (reversible airflow limitation) -controlled Post-BD increase in FEV 1 ≥ 12% and 400 ml from baseline (marked reversibility) High probability of asthma

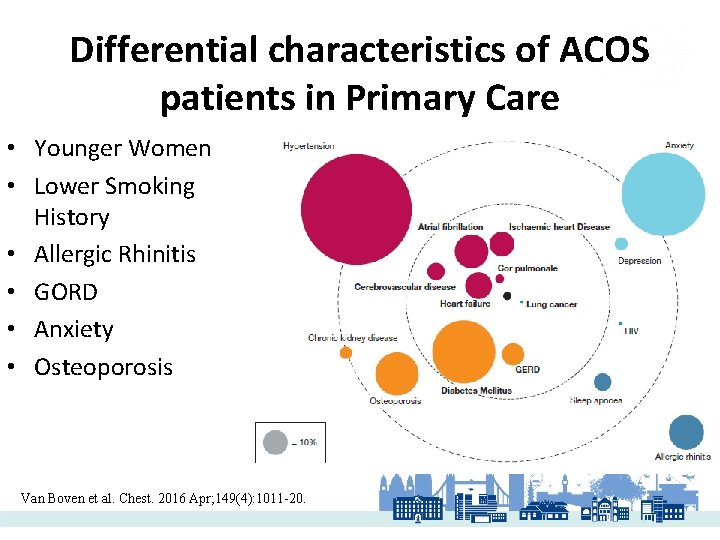
Differential characteristics of ACOS patients in Primary Care • Younger Women • Lower Smoking History • Allergic Rhinitis • GORD • Anxiety • Osteoporosis Van Boven et al. Chest. 2016 Apr; 149(4): 1011 -20.

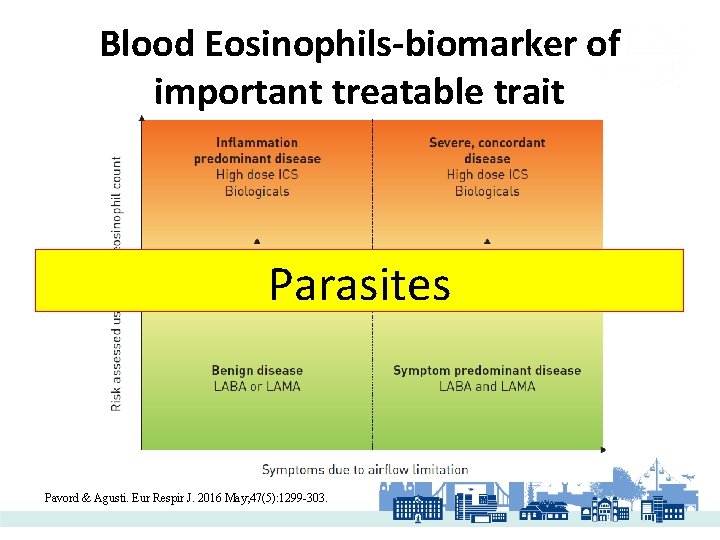
Blood Eosinophils-biomarker of important treatable trait Parasites Pavord & Agusti. Eur Respir J. 2016 May; 47(5): 1299 -303.

NICE 2010 • Additional Investigations: – CXR – FBC – BMI • Consider alternative diagnoses in: – older people without typical symptoms of COPD where the FEV 1/FVC ratio is < 0. 7 – younger people with symptoms of COPD where the FEV 1/FVC ratio is >0. 7

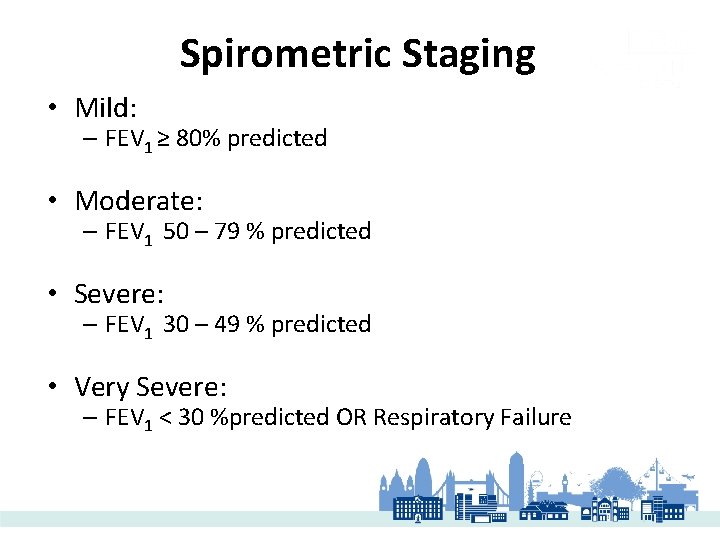
Spirometric Staging • Mild: – FEV 1 ≥ 80% predicted • Moderate: – FEV 1 50 – 79 % predicted • Severe: – FEV 1 30 – 49 % predicted • Very Severe: – FEV 1 < 30 %predicted OR Respiratory Failure

Severity Is Not All About Spirometry • Increased mortality with BMI < 21 • Exacerbations associated with significant morbidity and mortality • (m)MRC Scale of functional dyspnoea – (0) 1 Not troubled by breathlessness except on strenuous exercise – (1) 2 Short of breath when hurrying or walking up a slight hill – (2) 3 Walks slower than peers on level ground because of breathlessness, or has to stop for breath when walking at own pace – (3) 4 Stops for breath after walking about 100 m or after a few minutes on level ground – (4) 5 Too breathless to leave the house, or breathless when dressing or undressing

COPD Assessment Test


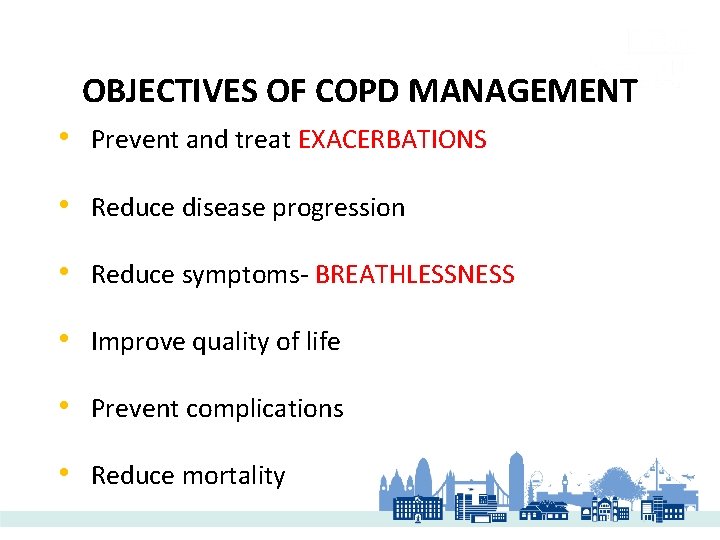
OBJECTIVES OF COPD MANAGEMENT • Prevent and treat EXACERBATIONS • Reduce disease progression • Reduce symptoms- BREATHLESSNESS • Improve quality of life • Prevent complications • Reduce mortality

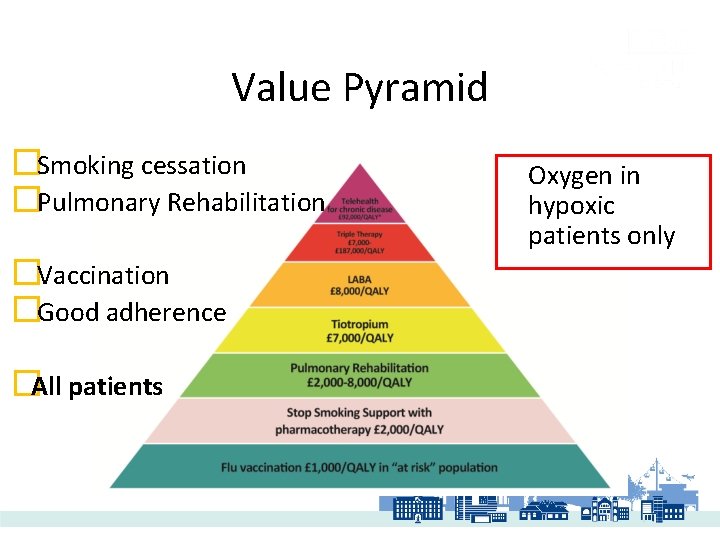
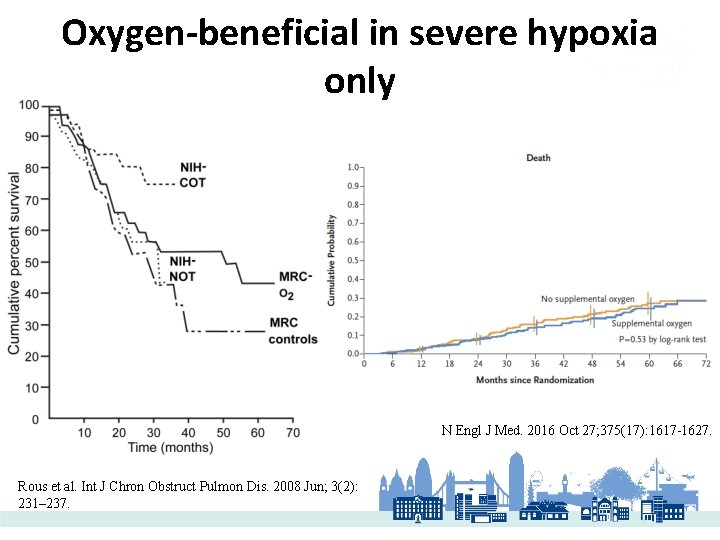
Value Pyramid �Smoking cessation �Pulmonary Rehabilitation �Vaccination �Good adherence �All patients Oxygen in hypoxic patients only

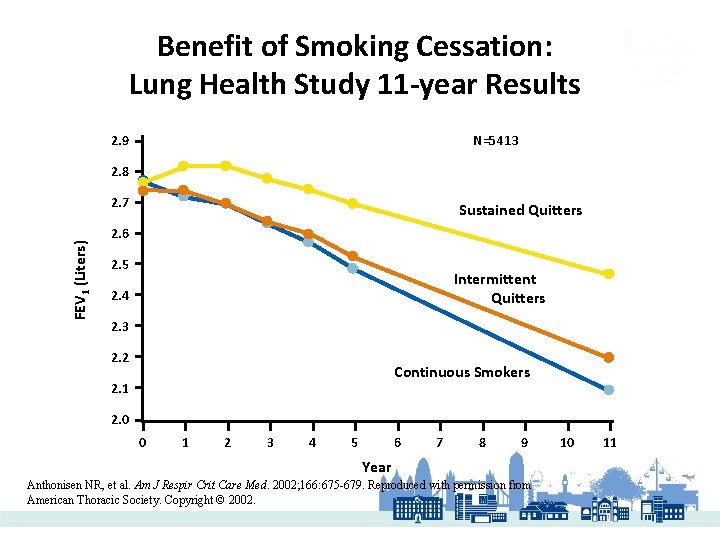
Benefit of Smoking Cessation: Lung Health Study 11 -year Results 2. 9 N=5413 2. 8 FEV 1 (Liters) 2. 7 Sustained Quitters 2. 6 2. 5 Intermittent Quitters 2. 4 2. 3 2. 2 Continuous Smokers 2. 1 2. 0 0 1 2 3 4 5 6 7 8 9 Year Anthonisen NR, et al. Am J Respir Crit Care Med. 2002; 166: 675 -679. Reproduced with permission from American Thoracic Society. Copyright © 2002. 10 11

NICE – Stop Smoking – Encouraging patients with COPD to stop smoking is one of the most important components of their management – All COPD patients still smoking, regardless of age, should be encouraged to stop, and offered help to do so, at every opportunity – Record a smoking history, including pack years smoked – Offer nicotine replacement therapy, varenicline or bupropion (unless contraindicated) combined with a support programme to optimise quit rates [2010]

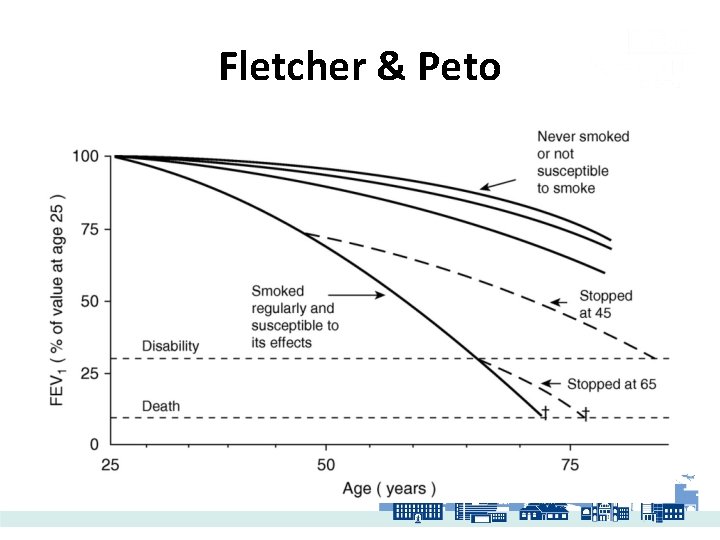
Fletcher & Peto

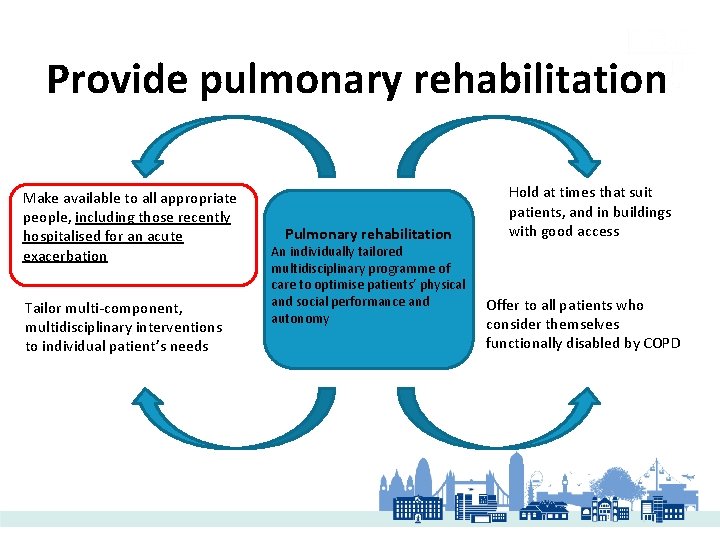
Provide pulmonary rehabilitation Make available to all appropriate people, including those recently hospitalised for an acute exacerbation Tailor multi-component, multidisciplinary interventions to individual patient’s needs Pulmonary rehabilitation An individually tailored multidisciplinary programme of care to optimise patients’ physical and social performance and autonomy Hold at times that suit patients, and in buildings with good access Offer to all patients who consider themselves functionally disabled by COPD

Medical Management

Choice of inhaler Device • Assess patients response to treatment using CAT • Choose inhaler that on an individual basis to suit patients needs and ability to use device • Consider using In-check device to identify suitable inhaler device – Low inspiratory effort Soft mist. MDI – Better inspiratory efforts DPI

In-Check device • The In-Check is a portable inspiratory flow meter that monitors both the disease and the response to treatment. It is designed for clinical and in-home use by both children and adults. • Each In-Check is individually calibrated to ensure a high degree of accuracy (+/- 10% or 10 L/min - whichever is greater • https: //www. foursquare-healthcare. co. uk/in-check-dial-g 16 inhaler-training-device/ • https: //www. youtube. com/watch? v=b. GCf. CGw 9 h 24 • https: //pharmacyinpractice. scot/2016/04/29/the-new-incheck-dial-has-arrived/

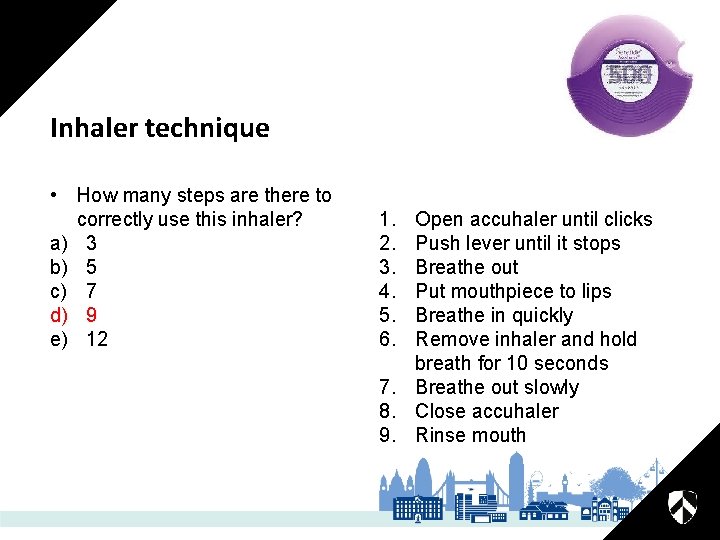
Inhaler technique • How many steps are there to correctly use this inhaler? a) b) c) d) e) 3 5 7 9 12

Inhaler technique • How many steps are there to correctly use this inhaler? a) 3 b) 5 c) 7 d) 9 e) 12 1. 2. 3. 4. 5. 6. Open accuhaler until clicks Push lever until it stops Breathe out Put mouthpiece to lips Breathe in quickly Remove inhaler and hold breath for 10 seconds 7. Breathe out slowly 8. Close accuhaler 9. Rinse mouth

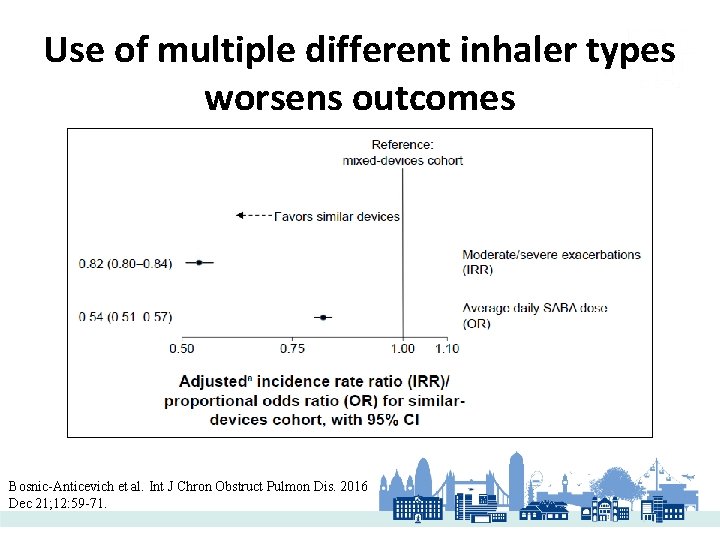
Use of multiple different inhaler types worsens outcomes Bosnic-Anticevich et al. Int J Chron Obstruct Pulmon Dis. 2016 Dec 21; 12: 59 -71.

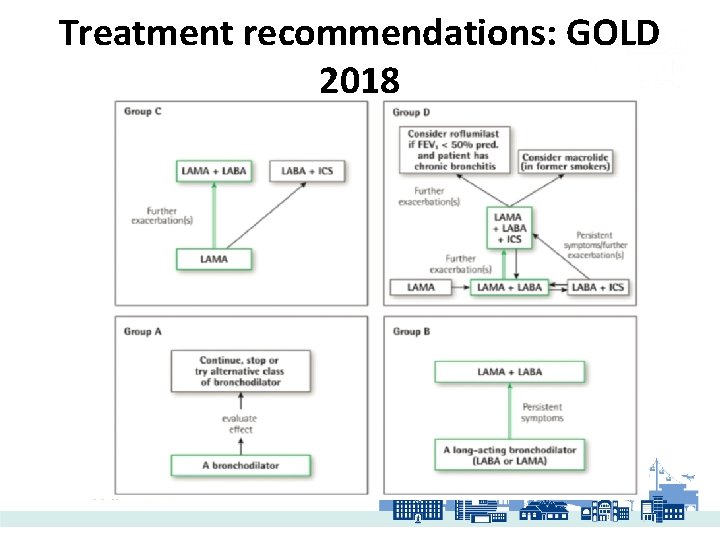
Treatment recommendations: GOLD 2018

SOME OF THE EVIDENCE…. • LABA/LAMA • ICS/LABA/LAMA

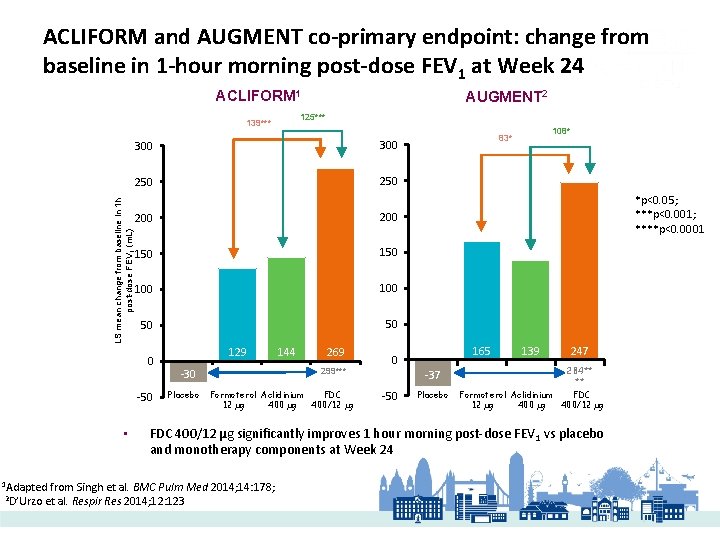
ACLIFORM and AUGMENT co-primary endpoint: change from baseline in 1 -hour morning post-dose FEV 1 at Week 24 ACLIFORM 1 125*** 139*** 300 250 LS mean change from baseline in 1 h post-dose FEV 1 (m. L) 300 200 150 100 50 50 0 -50 • 1 Adapted 2 D’Urzo AUGMENT 2 129 269 299*** -30 Placebo 144 Formoterol Aclidinium FDC 12 µg 400/12 µg *p<0. 05; ***p<0. 001; ****p<0. 0001 165 0 -37 -50 108* 83* Placebo 139 247 284** ** Formoterol Aclidinium FDC 12 µg 400/12 µg FDC 400/12 µg significantly improves 1 hour morning post-dose FEV 1 vs placebo and monotherapy components at Week 24 from Singh et al. BMC Pulm Med 2014; 14: 178; et al. Respir Res 2014; 12: 123

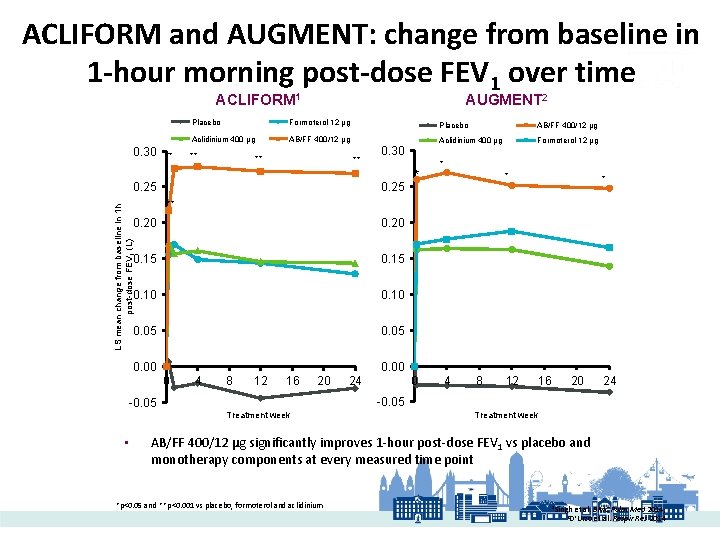
ACLIFORM and AUGMENT: change from baseline in 1 -hour morning post-dose FEV 1 over time ACLIFORM 1 0. 30 ** AUGMENT 2 Placebo Formoterol 12 µg Placebo AB/FF 400/12 µg Aclidinium 400 µg Formoterol 12 µg ** ** ** 0. 25 * * LS mean change from baseline in 1 h post-dose FEV 1 (L) ** 0. 20 0. 15 0. 10 0. 05 0. 00 0 -0. 05 • 0. 30 4 8 12 16 20 0 24 4 8 12 16 20 24 -0. 05 Treatment week AB/FF 400/12 µg significantly improves 1 -hour post-dose FEV 1 vs placebo and monotherapy components at every measured time point *p<0. 05 and **p<0. 001 vs placebo, formoterol and aclidinium 1 Singh et al. BMC Pulm Med 2014; et al. Respir Res 2014 2 D’Urzo

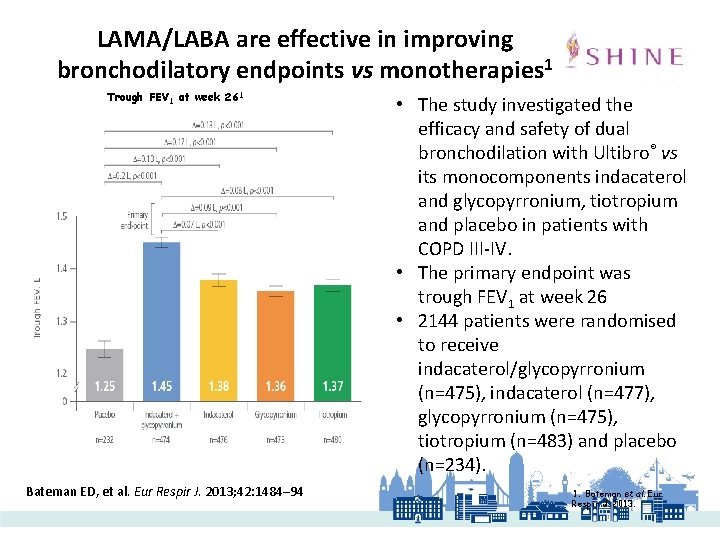
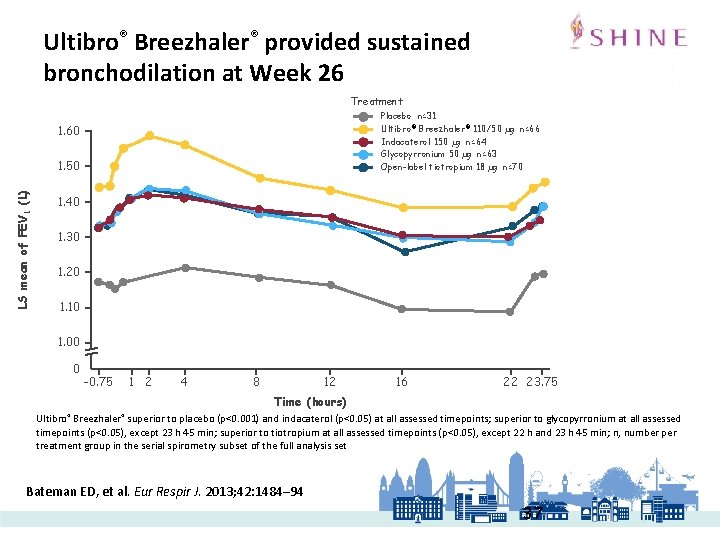
LAMA/LABA are effective in improving bronchodilatory endpoints vs monotherapies 1 Trough FEV 1 at week 261 Bateman ED, et al. Eur Respir J. 2013; 42: 1484– 94 • The study investigated the efficacy and safety of dual bronchodilation with Ultibro® vs its monocomponents indacaterol and glycopyrronium, tiotropium and placebo in patients with COPD III-IV. • The primary endpoint was trough FEV 1 at week 26 • 2144 patients were randomised to receive indacaterol/glycopyrronium (n=475), indacaterol (n=477), glycopyrronium (n=475), tiotropium (n=483) and placebo (n=234). 1. Bateman et al. Eur Respir J. 2013.

Ultibro® Breezhaler® provided sustained bronchodilation at Week 26 Treatment Placebo n=31 Ultibro ® Breezhaler® 110/50 µg n=66 Indacaterol 150 µg n=64 Glycopyrronium 50 µg n=63 Open-label tiotropium 18 µg n=70 1. 60 LS mean of FEV 1 (L) 1. 50 1. 40 1. 30 1. 20 1. 10 1. 00 0 -0. 75 1 2 4 8 12 16 22 23. 75 Time (hours) Ultibro® Breezhaler® superior to placebo (p<0. 001) and indacaterol (p<0. 05) at all assessed timepoints; superior to glycopyrronium at all assessed timepoints (p<0. 05), except 23 h 45 min; superior to tiotropium at all assessed timepoints (p<0. 05), except 22 h and 23 h 45 min; n, number per treatment group in the serial spirometry subset of the full analysis set Bateman ED, et al. Eur Respir J. 2013; 42: 1484– 94 37

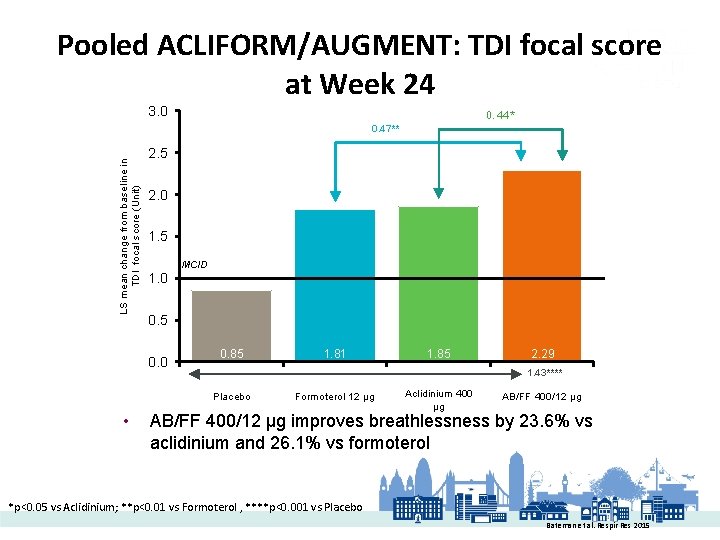
Pooled ACLIFORM/AUGMENT: TDI focal score at Week 24 3. 0 0. 44* LS mean change from baseline in TDI focal score (Unit) 0. 47** 2. 5 2. 0 1. 5 MCID 1. 0 0. 5 0. 0 0. 85 1. 85 2. 29 1. 43**** Placebo • 1. 81 Formoterol 12 µg Aclidinium 400 µg AB/FF 400/12 µg improves breathlessness by 23. 6% vs aclidinium and 26. 1% vs formoterol *p<0. 05 vs Aclidinium; **p<0. 01 vs Formoterol , ****p<0. 001 vs Placebo Bateman et al. Respir Res 2015

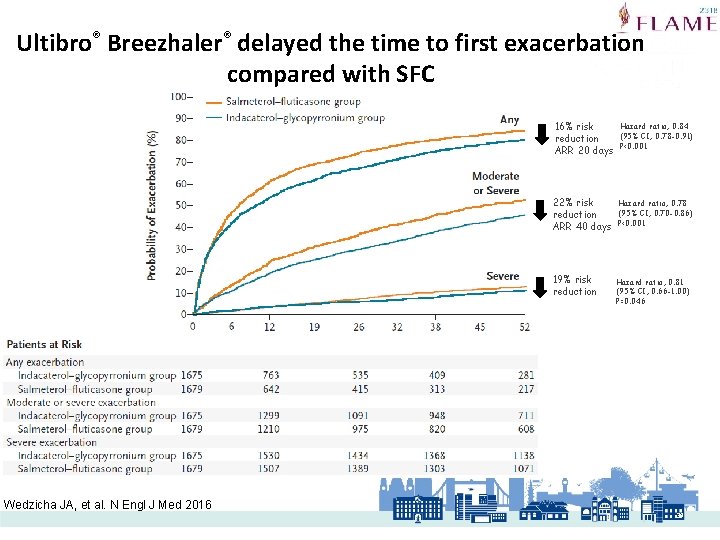
Ultibro® Breezhaler® delayed the time to first exacerbation compared with SFC Wedzicha JA, et al. N Engl J Med 2016 16% risk reduction ARR 20 days Hazard ratio, 0. 84 (95% CI, 0. 78 -0. 91) P<0. 001 22% risk reduction ARR 40 days Hazard ratio, 0. 78 (95% CI, 0. 70 -0. 86) P<0. 001 19% risk reduction Hazard ratio, 0. 81 (95% CI, 0. 66 -1. 00) P=0. 046 39

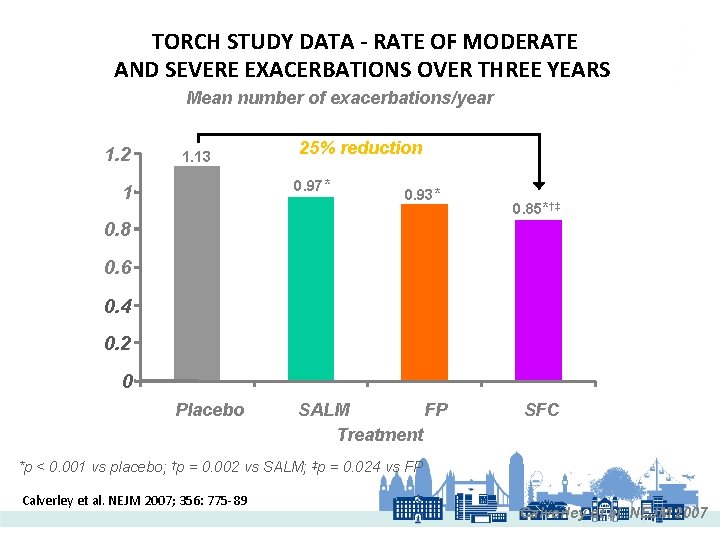
TORCH STUDY DATA - RATE OF MODERATE AND SEVERE EXACERBATIONS OVER THREE YEARS Mean number of exacerbations/year 1. 2 1. 13 25% reduction 0. 97* 1 0. 93* 0. 85*†‡ 0. 8 0. 6 0. 4 0. 2 0 Placebo SALM FP Treatment SFC *p < 0. 001 vs placebo; †p = 0. 002 vs SALM; ‡p = 0. 024 vs FP Calverley et al. NEJM 2007; 356: 775 -89 Calverley et al. NEJM 2007

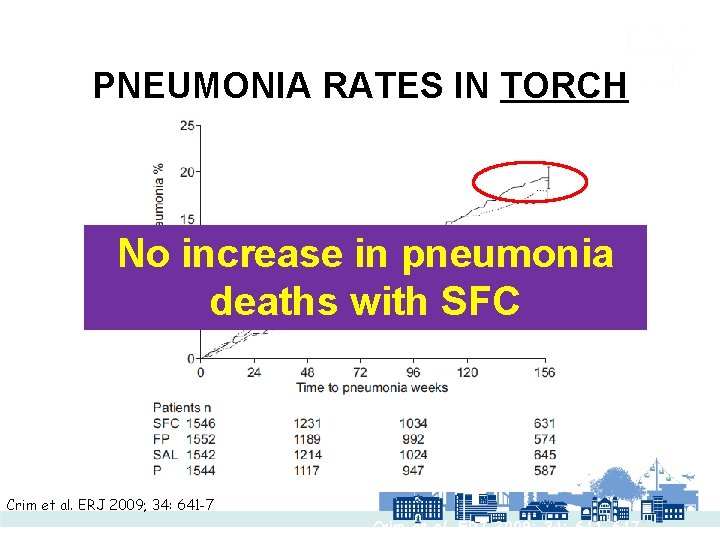
PNEUMONIA RATES IN TORCH No increase in pneumonia deaths with SFC Crim et al. ERJ 2009; 34: 641 -7 Crim et al. ERJ 2009; 34: 641 -647

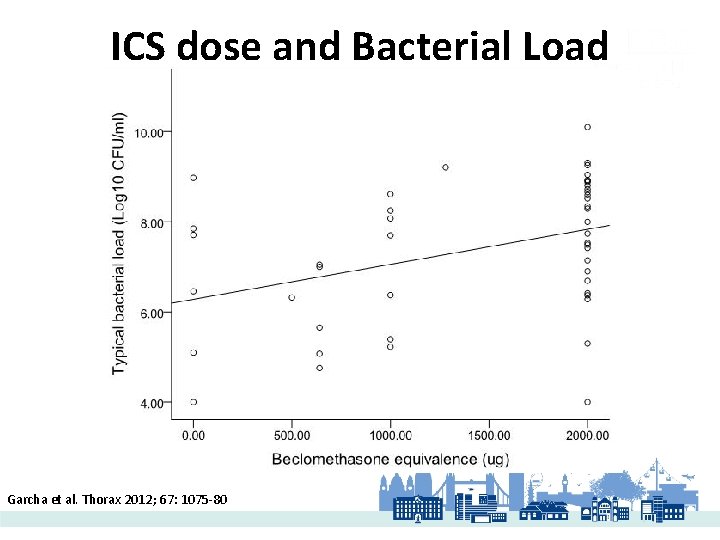
ICS dose and Bacterial Load Garcha et al. Thorax 2012; 67: 1075 -80

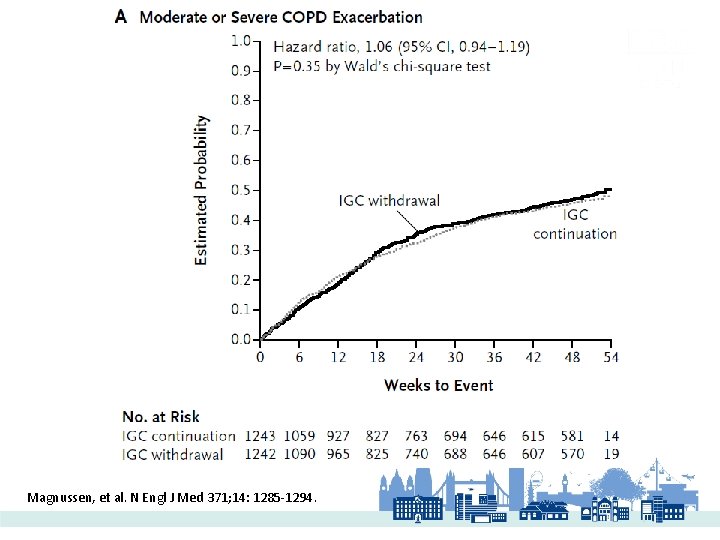
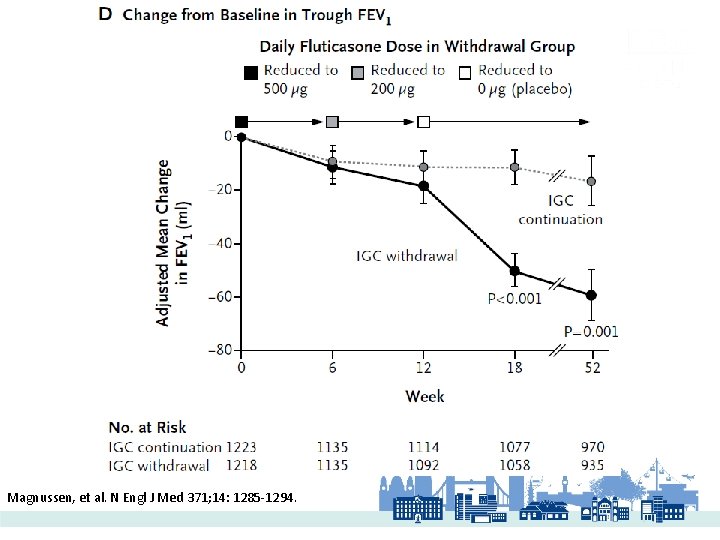
WISDOM 2014 Magnussen, et al. N Engl J Med 371; 14: 1285 -1294.

Magnussen, et al. N Engl J Med 371; 14: 1285 -1294.

Magnussen, et al. N Engl J Med 371; 14: 1285 -1294.

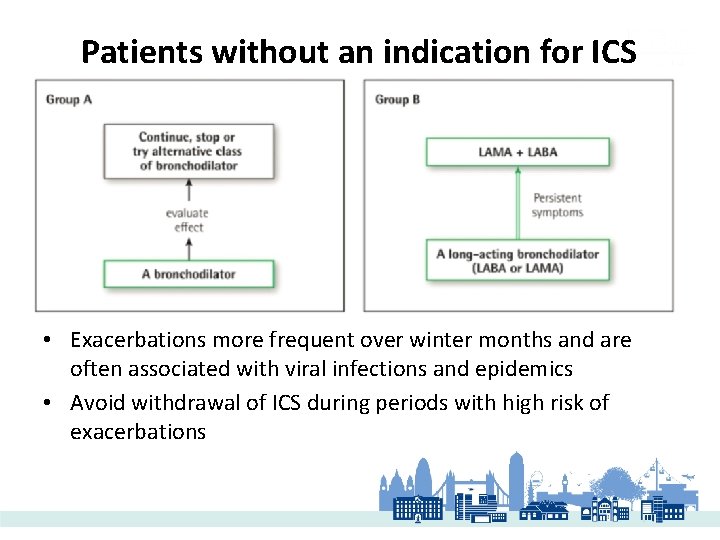
Patients without an indication for ICS • Exacerbations more frequent over winter months and are often associated with viral infections and epidemics • Avoid withdrawal of ICS during periods with high risk of exacerbations

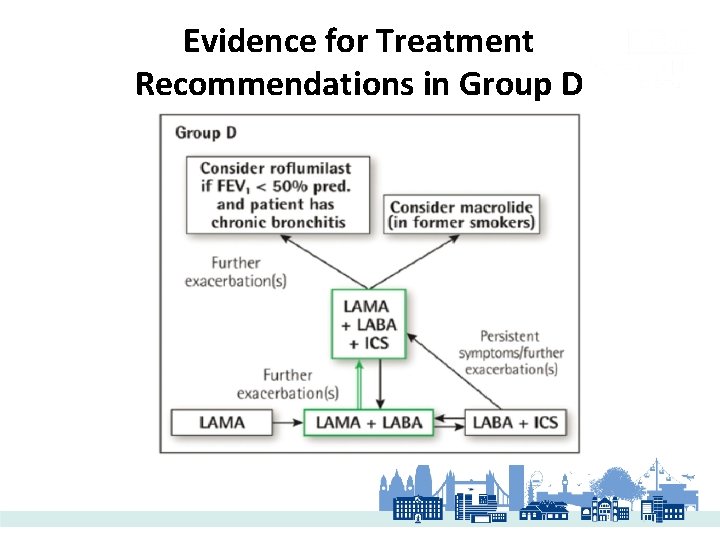
Evidence for Treatment Recommendations in Group D

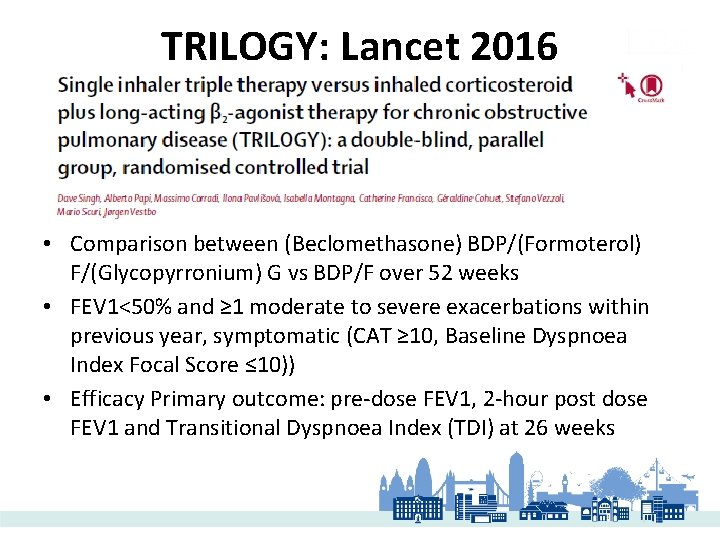
TRILOGY: Lancet 2016 • Comparison between (Beclomethasone) BDP/(Formoterol) F/(Glycopyrronium) G vs BDP/F over 52 weeks • FEV 1<50% and ≥ 1 moderate to severe exacerbations within previous year, symptomatic (CAT ≥ 10, Baseline Dyspnoea Index Focal Score ≤ 10)) • Efficacy Primary outcome: pre-dose FEV 1, 2 -hour post dose FEV 1 and Transitional Dyspnoea Index (TDI) at 26 weeks

• Both pre-dose FEV 1 and 2 -hour post dose FEV 1 change from baseline (latter above) were significantly higher at week 26 • TDI focal score improved at week 26 in both groups, but not significantly different between two treatment arms

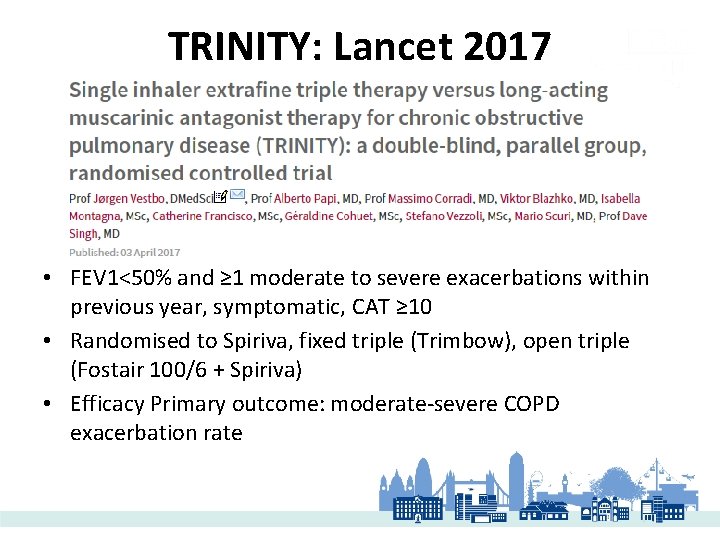
TRINITY: Lancet 2017 • FEV 1<50% and ≥ 1 moderate to severe exacerbations within previous year, symptomatic, CAT ≥ 10 • Randomised to Spiriva, fixed triple (Trimbow), open triple (Fostair 100/6 + Spiriva) • Efficacy Primary outcome: moderate-severe COPD exacerbation rate



TRIBUTE: Lancet 2018 • Comparison between (Beclomethasone) BDP/(Formoterol) F/(Glycopyrronium) G vs Indacaterol (IND)/G • FEV 1<50% and ≥ 1 moderate exacerbations within previous year, symptomatic (CAT ≥ 10) on any combination dual inhaled therapy • Primary outcome: rate of moderate-severe COPD exacerbations over 52 weeks

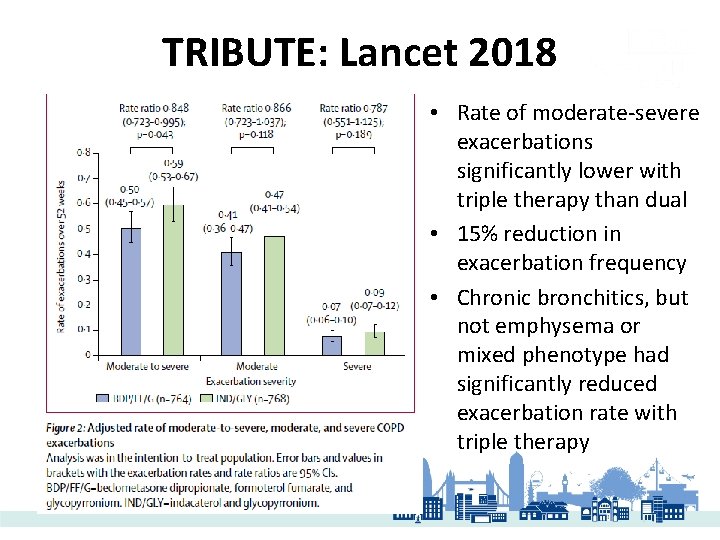
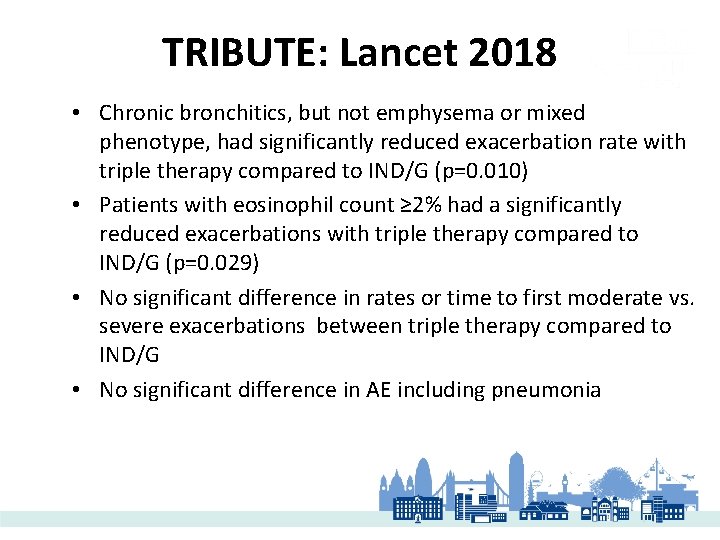
TRIBUTE: Lancet 2018 • Rate of moderate-severe exacerbations significantly lower with triple therapy than dual • 15% reduction in exacerbation frequency • Chronic bronchitics, but not emphysema or mixed phenotype had significantly reduced exacerbation rate with triple therapy

TRIBUTE: Lancet 2018 • Chronic bronchitics, but not emphysema or mixed phenotype, had significantly reduced exacerbation rate with triple therapy compared to IND/G (p=0. 010) • Patients with eosinophil count ≥ 2% had a significantly reduced exacerbations with triple therapy compared to IND/G (p=0. 029) • No significant difference in rates or time to first moderate vs. severe exacerbations between triple therapy compared to IND/G • No significant difference in AE including pneumonia

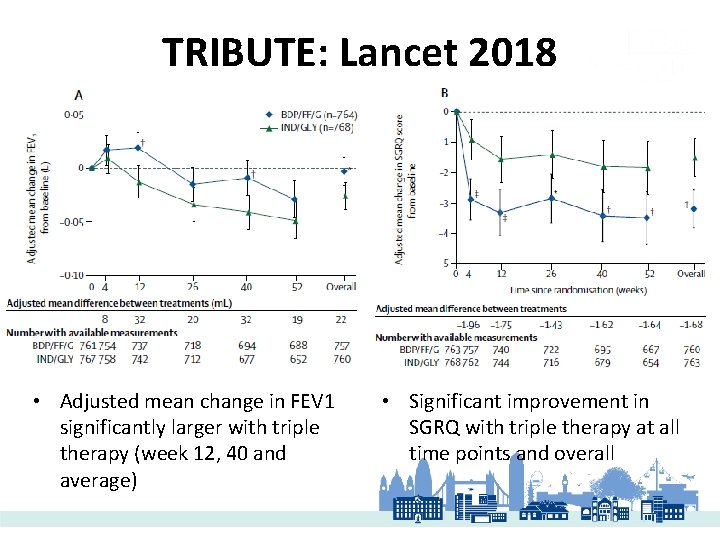
TRIBUTE: Lancet 2018 • Adjusted mean change in FEV 1 significantly larger with triple therapy (week 12, 40 and average) • Significant improvement in SGRQ with triple therapy at all time points and overall

IMPACT: NEJM 2018 • Fluticasone (FF)/Umeclidinium (U)/Vilanterol (V) vs. U/V over 52 weeks usingle Ellipta device • Symptomatic (CAT ≥ 10), FEV 1<50% and ≥ 1 moderate or severe exacerbation or FEV 1 50 -80% ≥ 2 moderate or severe exacerbation • Primary outcome – annual rate of moderate or severe COPD exacerbation

IMPACT: NEJM 2018

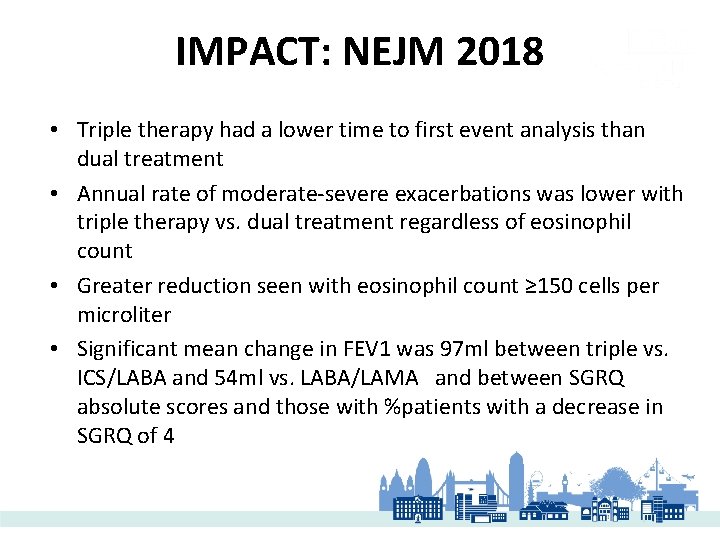
IMPACT: NEJM 2018 • Triple therapy had a lower time to first event analysis than dual treatment • Annual rate of moderate-severe exacerbations was lower with triple therapy vs. dual treatment regardless of eosinophil count • Greater reduction seen with eosinophil count ≥ 150 cells per microliter • Significant mean change in FEV 1 was 97 ml between triple vs. ICS/LABA and 54 ml vs. LABA/LAMA and between SGRQ absolute scores and those with %patients with a decrease in SGRQ of 4

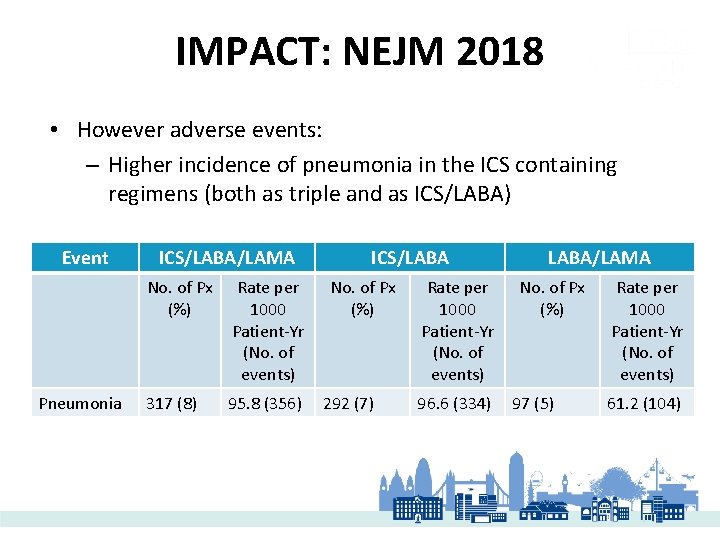
IMPACT: NEJM 2018 • However adverse events: – Higher incidence of pneumonia in the ICS containing regimens (both as triple and as ICS/LABA) Event Pneumonia ICS/LABA/LAMA No. of Px (%) Rate per 1000 Patient-Yr (No. of events) 317 (8) 95. 8 (356) ICS/LABA No. of Px (%) 292 (7) Rate per 1000 Patient-Yr (No. of events) 96. 6 (334) LABA/LAMA No. of Px (%) 97 (5) Rate per 1000 Patient-Yr (No. of events) 61. 2 (104)

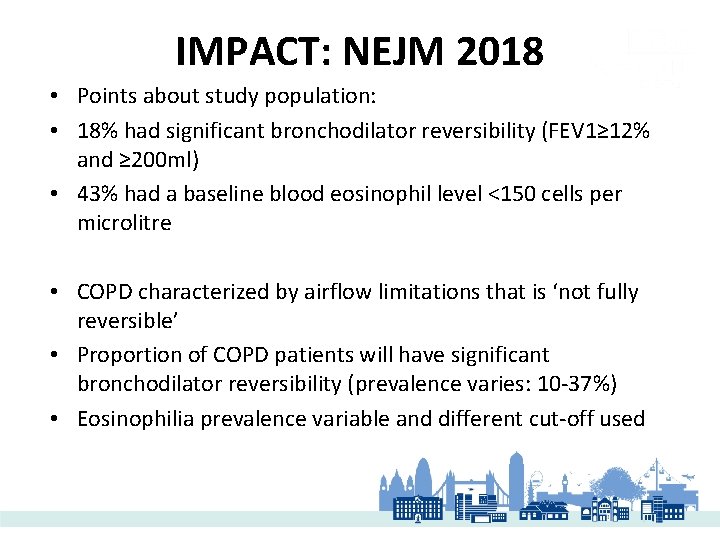
IMPACT: NEJM 2018 • Points about study population: • 18% had significant bronchodilator reversibility (FEV 1≥ 12% and ≥ 200 ml) • 43% had a baseline blood eosinophil level <150 cells per microlitre • COPD characterized by airflow limitations that is ‘not fully reversible’ • Proportion of COPD patients will have significant bronchodilator reversibility (prevalence varies: 10 -37%) • Eosinophilia prevalence variable and different cut-off used

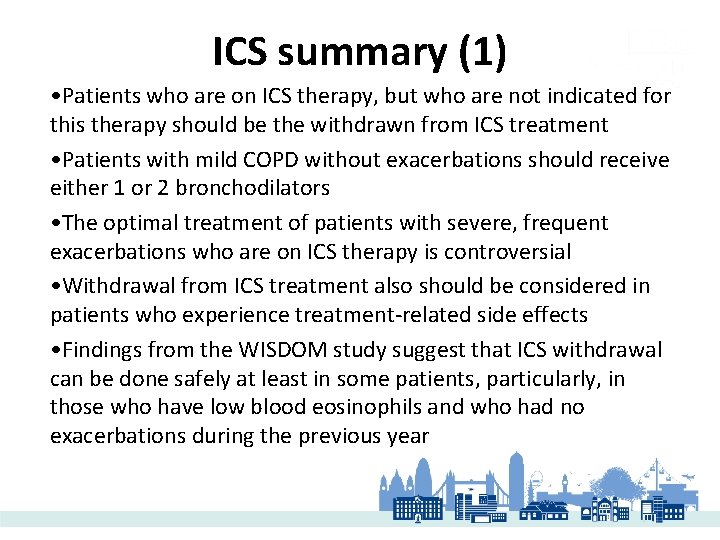
ICS summary (1) • Patients who are on ICS therapy, but who are not indicated for this therapy should be the withdrawn from ICS treatment • Patients with mild COPD without exacerbations should receive either 1 or 2 bronchodilators • The optimal treatment of patients with severe, frequent exacerbations who are on ICS therapy is controversial • Withdrawal from ICS treatment also should be considered in patients who experience treatment-related side effects • Findings from the WISDOM study suggest that ICS withdrawal can be done safely at least in some patients, particularly, in those who have low blood eosinophils and who had no exacerbations during the previous year

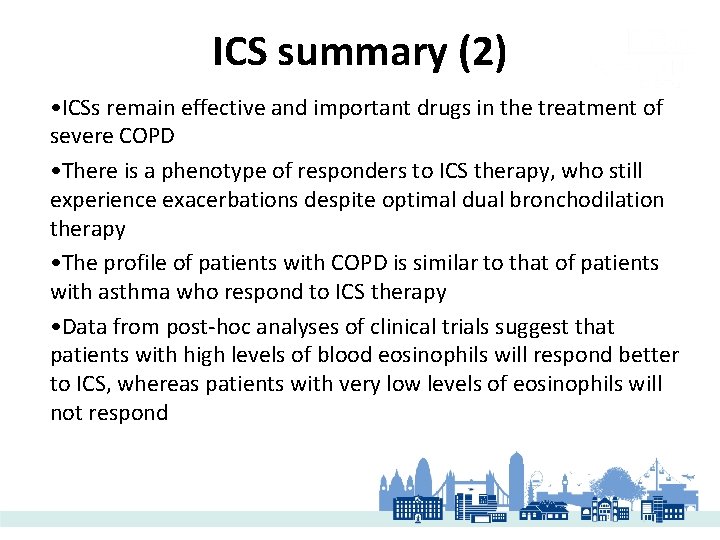
ICS summary (2) • ICSs remain effective and important drugs in the treatment of severe COPD • There is a phenotype of responders to ICS therapy, who still experience exacerbations despite optimal dual bronchodilation therapy • The profile of patients with COPD is similar to that of patients with asthma who respond to ICS therapy • Data from post-hoc analyses of clinical trials suggest that patients with high levels of blood eosinophils will respond better to ICS, whereas patients with very low levels of eosinophils will not respond

Oxygen-beneficial in severe hypoxia only N Engl J Med. 2016 Oct 27; 375(17): 1617 -1627. Rous et al. Int J Chron Obstruct Pulmon Dis. 2008 Jun; 3(2): 231– 237.

Questions?