Coordination complexes Lector Varikova T O Complex compounds











- Slides: 15

Coordination complexes Lector Varikova T. O.

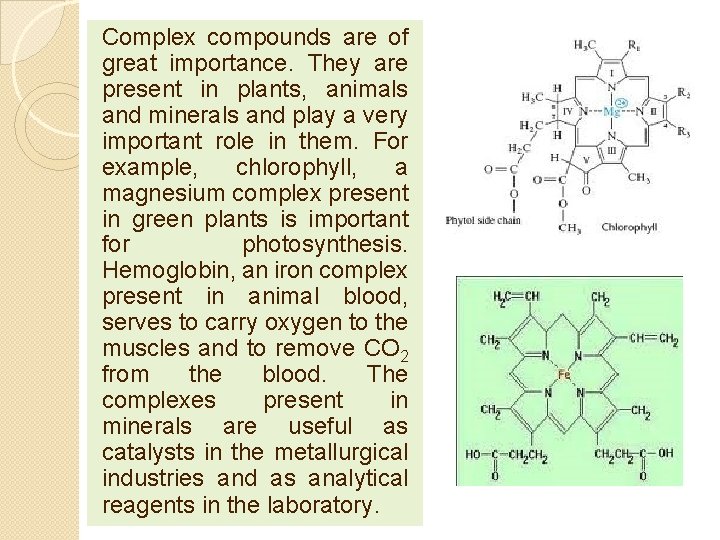
Complex compounds are of great importance. They are present in plants, animals and minerals and play a very important role in them. For example, chlorophyll, a magnesium complex present in green plants is important for photosynthesis. Hemoglobin, an iron complex present in animal blood, serves to carry oxygen to the muscles and to remove CO 2 from the blood. The complexes present in minerals are useful as catalysts in the metallurgical industries and as analytical reagents in the laboratory.

Coordination complexes of transition metals were studied by the Swiss chemist Alfred Werner around the turn of the century. His work led to modern theories of coordination chemistry. In 1913 Werner recived the Nobel Prize in chemistry for this work. Compounds are called complex when there are complexes at the points of their crystals that are capable of an independent existence in solution.

Werner′s theory: According to Werner′s theory a metal ion has two types of valencies: the primary valency and the secondary valency. Primary valency is the ionic valency and it is equal to the positive charge of the cation. The negative ions bound by this valency are liberated on ionization of the complex compound. The anions or neutral molecules bound to the transition metal ions by secondary valency are not ionisable.

Primary valency is statisfied by cations and anions only, while secondary valencies are satisfied by anions or neutral ligands molecules. The central metal ion with its ligands is called the coordination sphere. The coordination sphere in a formula of coordination complex is enclosed in brackets [ ].

In a coordination sphere, ligands are bonded to the central metal atom by coordinate covalent bonds. The number of coordinate covalent bonds that link the central atom or ion to its ligands in a coordination sphere is called the coordination number for the complex. This number equals the number of ligands in the coordination sphere if each ligand is attached to the central atom by only one coordinate covalent bond.

The classification of complexes Complexes are classified as cation, and neutral, depending of their electrical charge. In an approximation of an ionic model the charge of a complex is the algebraic sum of the charges of the particles forming it. Cation complexes are formed by coordination of neutral molecules (H 2 O, NH 3, CO et cetera) around positive ions. [Al(H 2 O 6]Cl 3 [Zn(NH 3)4]Cl 2

In anion complexes the role of complexing agent is played by an atom with a positive oxidation state, while the ligands are atoms with a negative oxidation state. K[Al(OH)4] K 3[Fe(CN)6] K 2[Be. F 4] Neutral complexes are formed through the coordination of molecules around an atom and also through the simultaneous coordination of negative ions and molecules around the central metal ion. [Pt(NH ) Cl ] [Ni(CO) ]

Types of ligands Ligands can be classified according to the number of coordinate covalent bonds they form with a metal atom or ion. Ligands such as NH 3 and CN-, which coordinate by only one donor atom, are called monodentate ligands. Other common monodentate ligands are H 2 O, CO, OH-, Cl, Br-, SCN-. Ligands that coordinate to a metal ion by two or more donor atoms are called polydentate ligands. A ligand with two donor atoms is a bidentate ligand, a ligand with three donor atoms is a terdentate ligand, and a ligand with four donor atoms is a tetradentate ligand.

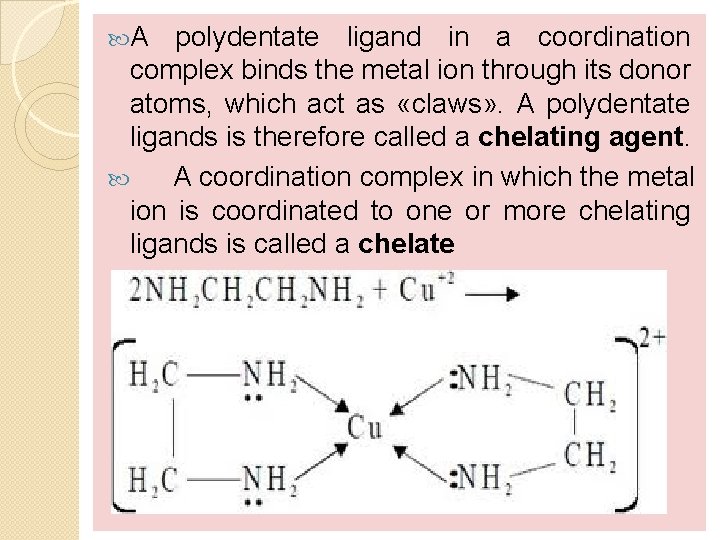
A polydentate ligand in a coordination complex binds the metal ion through its donor atoms, which act as «claws» . A polydentate ligands is therefore called a chelating agent. A coordination complex in which the metal ion is coordinated to one or more chelating ligands is called a chelate

Names and Formulas of Common Ligands

Naming Coordination Complexes A coordination compound that consists of cations and anions is named like a simple salt: the cation is named first and the anion second. Neutral coordination compounds are named like complex cations. In the name of a complex cation, the ligands are named first, then the metal. The oxidation state of the metal is written with a Roman numeral enclosed if parentheses after the name of the metal. The names of the ligands, the

The names of negative ligands end in –o. Most neutral ligands are named as molecules. Two important exceptions are water and ammonia, which are named aqua and ammine, respectively. If a complex ion contains two or more identical ligands, their number is indicated by a Greek prefix di -, tri-, tetra-, penta-, hexa-, and so on. When two or more different ligands are in the coordination sphere, their names are listed in alphabetical order, Examples: [Cu(H 2 O)4]2+ tetraaquacopper (II) ion [Cr(NH 3)3 Br 3] – triamminetribromoc hromium(III) [Fe(H 2 O)5(SCN)]SO 4 – pentaaquathiocyano iron(III) silfate.

A complex anion is named by the same rules as a complex cation, except that the suffix – ate is added to the name of metal. Thus, the names of complex anions are analogous to the names of simple oxoanions sush as nitrate, carbonate, and sulfate. F. e. [Ni(CN)4]2 - tetracyanonickelate(II)ion. When the name of the metal ends with – um, as in chromium and platinum, the suffix – ate is added to the stem of Cr(CN)6]3 - hexcyanochromate(III ) ion [Pt. Cl 4]2 - tetrachloroplatinate(II) ion.

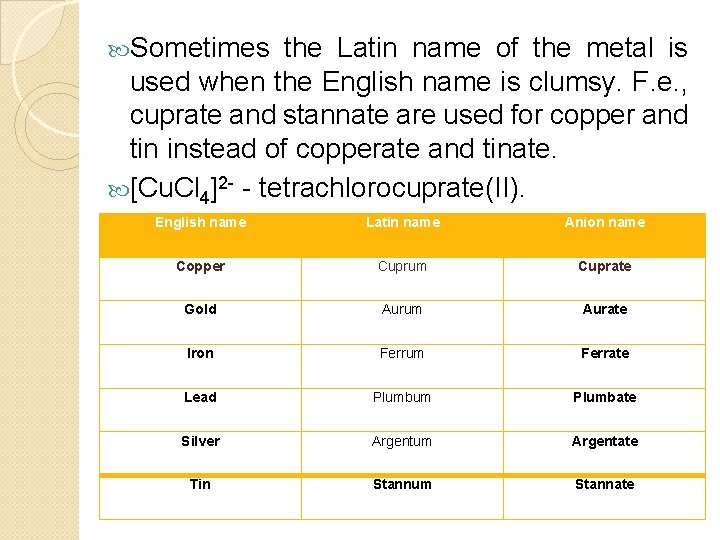
Sometimes the Latin name of the metal is used when the English name is clumsy. F. e. , cuprate and stannate are used for copper and tin instead of copperate and tinate. [Cu. Cl 4]2 - - tetrachlorocuprate(II). English name Latin name Anion name Copper Cuprum Cuprate Gold Aurum Aurate Iron Ferrum Ferrate Lead Plumbum Plumbate Silver Argentum Argentate Tin Stannum Stannate