Coordination Chemistry Most amazing field of inorganic chemistry








![[ Co (NH 3) 6 ] Cl 3 -Hexa-amine cobalt (lll) -Ch. Ioride In [ Co (NH 3) 6 ] Cl 3 -Hexa-amine cobalt (lll) -Ch. Ioride In](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-12.jpg)





![v. Eg: - The charge number of frrocyanide ion or hexacyanoferrate (ll). [Fe(CN)6]-4 is v. Eg: - The charge number of frrocyanide ion or hexacyanoferrate (ll). [Fe(CN)6]-4 is](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-23.jpg)




![Example. In ferrocyanide ion [Fe(CN)6]4 Fe ------ 2 e----> Fe 2+ + 6 CN Example. In ferrocyanide ion [Fe(CN)6]4 Fe ------ 2 e----> Fe 2+ + 6 CN](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-28.jpg)
![Metal Fe Complex [Fe(CN)6]3 - Z X Y EAN 26 -03 12 35 29 Metal Fe Complex [Fe(CN)6]3 - Z X Y EAN 26 -03 12 35 29](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-30.jpg)








- Slides: 51

Coordination Chemistry

Most amazing field of inorganic chemistry is coordination chemistry. v Transition metal possess characteristic property of undergoing complex formation. v Coordination compounds are also formed by several non –transitional metals. v The branch of chemistry which deals with the study of coordination compounds or complex compound is known as Coordination chemistry. v Coordination compounds are widely distributed in minerals, plants, animals , they are play important biological functions. v

# Chlorophyll is a coordination compound containing Mg. The red pigment hemoglobin present in blood, which is responsible for the regulation of respiration process in animal world, it is a coordination compound of iron. # A variety of metallurgical process, catalysts and analytical reagents make use of coordination compounds. # The systematic attempt of explaining the formation , reaction, structure of bonding of coordination compound was made by Alfred Werner.

v In this theory used two types of , linkages, such as primary and secondary by metal atom / ion in a coordination compound. v primary linkage is considered as ionic bond while secondary linkage is covalent bond. Coordination compound contain a central metal ion surrounded by, number of oppositely charged ions or neutral molecules. Eg: - tetra ammine copper(ll) ion, [Cu(NH 3)4]2+ v. In this complex copper is the central metal ion while ammonia is the neutral molecule.

Werner’s Theory of coordination compounds v. Basic Postulates: 1) Most of the metallic elements exhibits two types of valence. Primary valence and secondary valence. 2) Every metal tend to satisfy both valences. 3) Every metal has fixed number of secondary valence. 4) The secondary valence is always directed toward fixed position in space.

Salient features v. Principal valence is also known as , primary valence or ionisable valence, designated by solid line ( ____ ). In modern term it corresponds to the oxidation state of metal ion. v. The secondary valence is called as, non-ionisable or subsidiary valence. It is designated by dotted line (……. . ). v. There are two sphere of attraction around the metal ion in a complex, inner sphere and outer sphere.

v. Groups present in the inner sphere are , firmly attached to the metal ion , and therefore cannot be separated easily. v. The primary valences are those which a metal ion exercises towards the negative groups so that its normal charge is satisfied by the formation of simple salts. Primary valences of Co, Cu, Ag, are 3, 2, 1. to produces simple salts.

v. Secondary valences are satisfied by –ve ions or neutral molecules. v. Co has secondary valence is 6 , Co forms a complexes like, [ Co (NH 3) 6 ] 3+ [ Co (NH 3) 5 Cl ]2+ [ Co (NH 3) 4 Cl 2 ] + , [ Co (NH 3) 3 Cl 3] 0 v. Every element is characterized by fixed secondary valences. Fe(II), Co(III), Pt(IV). v. The primary valences are non rigid and non directional, while secondary valence, have directional properties.

v. Werner combined the interpretation of both , geometrical and optical isomerism. v. If the metal ion has, a secondary valence four, therefore geometry of the complex can be square planar or tetrahedral. v. If the metal ion has secondary valence six, the geometry of the complex may be octahedral.

Werner’s theory in the light of modern electronic theory of valence The combing capacity of a metal in form of two sphere of attraction or two zones of attraction. The first or inner zone surrounding the metal is called as coordination sphere. Neutral molecules or negatively charged, ligands are linked by coordinate bonds to the central metal ion in this zone. The linking is rigid and tight, this sphere functions as a non ionisable zone.

The second or outer zone, surrounding the coordination zone is called as ionization sphere. In this zone the metal ion satisfies it’s primary valence , by linking with negatively charged ions which get ionized on dissolving the complex in water. Werner introduced the square brackets [ ] to enclose the coordination sphere of the complex compound which contains the central metal ion and the ligands. The portion outside the square bracket contains ions of ionization sphere.
![Co NH 3 6 Cl 3 Hexaamine cobalt lll Ch Ioride In [ Co (NH 3) 6 ] Cl 3 -Hexa-amine cobalt (lll) -Ch. Ioride In](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-12.jpg)
[ Co (NH 3) 6 ] Cl 3 -Hexa-amine cobalt (lll) -Ch. Ioride In above example six ammonia molecules in coordination sphere and three chloride ions in ionization sphere. [Co(NH 3)5 Cl]Cl 2 - penta aminechloro cobalt(lll) - Chloride. Five ammonia molecules in coordination sphere and two chloride ions in ionization sphere The primary valence (oxidation state ) of Co is 3 while secondary valence is 6.

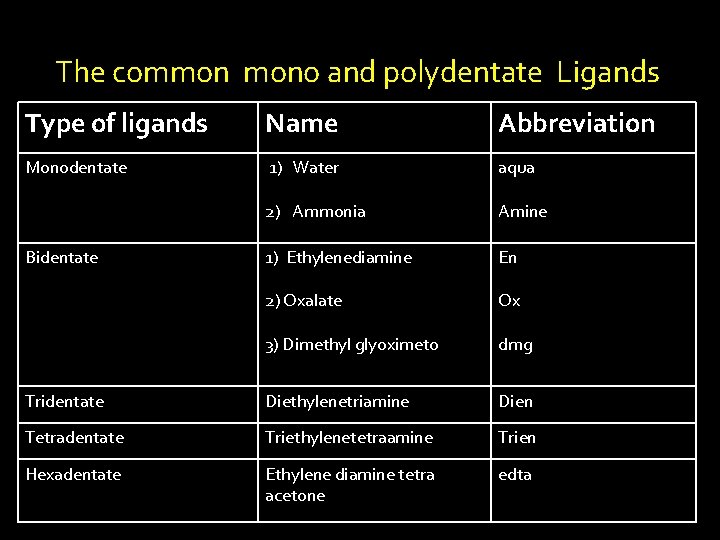
Ligands v. The molecules or ions are coordinated to the metal atom or ions in a coordination compound is called as ligands or donor groups. Classification of Ligands 1) Monodentate ligands 2) Poly dentate ligands

v. Mono or Unidentate Ligand: The ligand molecule / ion which has only one donor atom or , one point of attachment and can coordinate with metal ion , at only one site in a complex. eg. NH 3 molecule has one donor atom i. e nitrogen. Hence it can be linked to the metal of complex through nitrogen atom. v. Poly or Multidentate Ligands: Ligand molecule or ion which has more donor atoms or points of attachment and can be linked to same metal in a complex using more donating sites.

It is classified as bi, tri, …. . hexa dentate , it is depending upon the number of points attachments with the ligand. v. Polydentate ligands involving ring structures, are called as metal chelates and ligands as chelate ligands, and the process of forming metal chlete is called as chelation. . Eg. Ethylene diamine.

Ambidentate ligands: If has two or more donor atoms, a complex is formed only one donor atom is attached to the metal is called as ambidentate ligands. Eg. NO 2 - group has the donor atoms (N & O). Out of two only one donor atom are linked to metal as , M- ONO or M-NO 2.

The common mono and polydentate Ligands Type of ligands Name Abbreviation Monodentate 1) Water aqua 2) Ammonia Amine 1) Ethylenediamine En 2) Oxalate Ox 3) Dimethyl glyoximeto dmg Tridentate Diethylenetriamine Dien Tetradentate Triethylenetetraamine Trien Hexadentate Ethylene diamine tetra acetone edta Bidentate

Coordination number (Ligancy) v The number of ligands which are directly bonded to central metal atom or ion in a complex is called as coordination number. v The coordination number of metal ion is influenced by several factors such as: 1) 2) 3) 4) Charge on the metal ion Charge on the ligand The relative sizes of metal ion and ligand Forces of repulsion between ligand

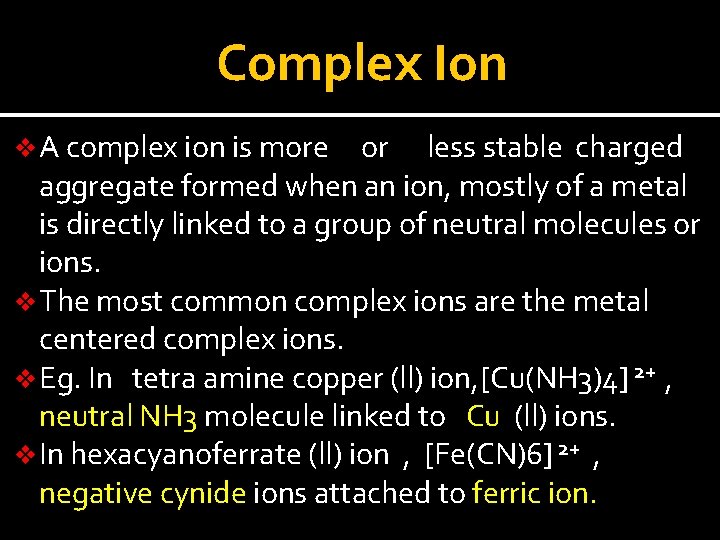
Complex Ion v A complex ion is more or less stable charged aggregate formed when an ion, mostly of a metal is directly linked to a group of neutral molecules or ions. v The most common complex ions are the metal centered complex ions. v Eg. In tetra amine copper (ll) ion, [Cu(NH 3)4] 2+ , neutral NH 3 molecule linked to Cu (ll) ions. v In hexacyanoferrate (ll) ion , [Fe(CN)6] 2+ , negative cynide ions attached to ferric ion.

Homoleptic and Hetroleptic complexes 1) Homoleptic complexes Complex in which a metal is attached to one kind of donor group are homoleptic complexes. Eg. In hexa amine cobalt ( lll) ion, [ Co (NH 3)6 ] 3+ six ammonia groups are attached to cobalt.

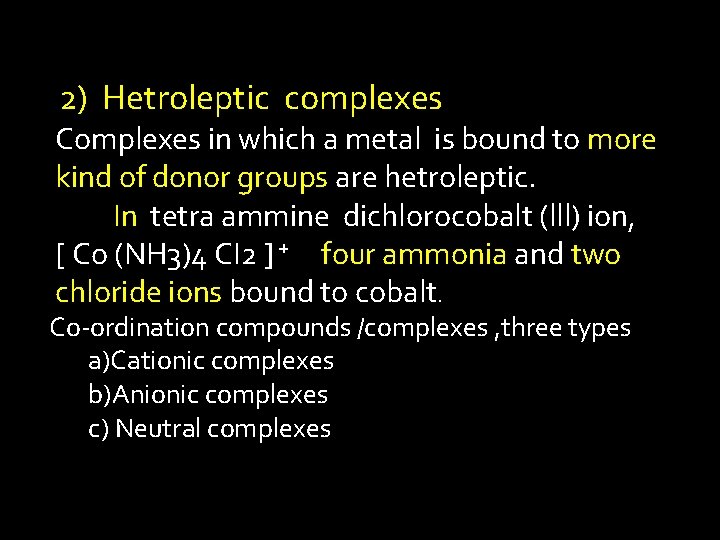
2) Hetroleptic complexes Complexes in which a metal is bound to more kind of donor groups are hetroleptic. In tetra ammine dichlorocobalt (lll) ion, [ Co (NH 3)4 CI 2 ] + four ammonia and two chloride ions bound to cobalt. Co-ordination compounds /complexes , three types a)Cationic complexes b)Anionic complexes c) Neutral complexes

Charge number of Complex ion v Net charge carried by complex ion is called its charge number. v It is equal to algebraic sum of charges carried out by central metal ion and ligands attached to it.
![v Eg The charge number of frrocyanide ion or hexacyanoferrate ll FeCN64 is v. Eg: - The charge number of frrocyanide ion or hexacyanoferrate (ll). [Fe(CN)6]-4 is](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-23.jpg)
v. Eg: - The charge number of frrocyanide ion or hexacyanoferrate (ll). [Fe(CN)6]-4 is -4. The charge number of [Fe(CN)6]-4 = charge on Fe+2 + 6 x charge on CN - ion. = ( + 2 ) + 6 (- 1 ) = -4

Difference between double salts and coordination compounds. 1) Double salts, are those molecular or addition compounds which exist in solid state but dissociate into their constituent ions when dissolved in water. v. Thus double salt lose their identity in aqueous solution. v. Eg. Mohr’s salt. It is a mixture of saturated sol. of ferrous sulphate and ammonium sulphate.

2) Coordination compounds, are those molecular or addition compounds which retain their identity in aqueous solutions and show properties entirely different from those of their constituent ions. Eg. Potassium ferrocynide.

Characteristics of complex ions The central metal ion in a complex is usually , a transition metal. 1) Exhibit multiple oxidation states. 2) small ionic size and high charge. 3) make available suitable orbital's of appropriate energy to accommodate the electron donated by the ligand. 4) Ones an ion forms a complex it losses its individual properties and acquires the properties which are the characteristic of the complex ion. 5) A complex ion retain its identity in solution may undergo dissociation.

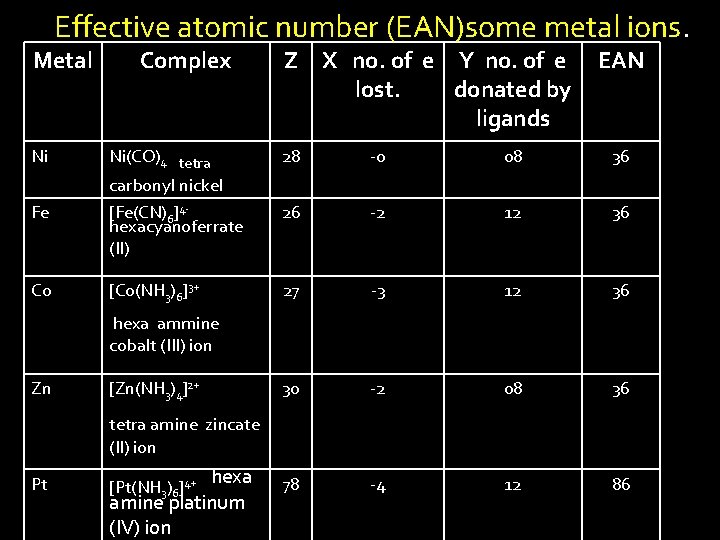
Effective atomic number (EAN) v It is a total number of electrons around central metal ion present in a complex and calculated as sum of electrons on metal ion and number of electron donated by ligands v EAN= Z - X + Y Where Z=atomic number of metal ion X = no. of electrons lost during the formation of the metal ion from its atom Y = no. of electrons donated by the ligands
![Example In ferrocyanide ion FeCN64 Fe 2 e Fe 2 6 CN Example. In ferrocyanide ion [Fe(CN)6]4 Fe ------ 2 e----> Fe 2+ + 6 CN](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-28.jpg)
Example. In ferrocyanide ion [Fe(CN)6]4 Fe ------ 2 e----> Fe 2+ + 6 CN 26 e 24 e + 6 x 2 = [Fe(CN)6]424 + 12 = 36 e EAN = 26 – 2 + 12 = 36 Here – 2 , no of electrons lost during formation of Fe ++

Effective atomic number (EAN)some metal ions. Metal Complex Z X no. of e Y no. of e lost. donated by ligands EAN Ni Ni(CO)4 tetra carbonyl nickel 28 -0 08 36 Fe [Fe(CN)6]4 hexacyanoferrate (II) 26 -2 12 36 Co [Co(NH 3)6]3+ 27 -3 12 36 30 -2 08 36 78 -4 12 86 hexa ammine cobalt (III) ion Zn [Zn(NH 3)4]2+ tetra amine zincate (II) ion Pt hexa amine platinum (IV) ion [Pt(NH 3)6]4+
![Metal Fe Complex FeCN63 Z X Y EAN 26 03 12 35 29 Metal Fe Complex [Fe(CN)6]3 - Z X Y EAN 26 -03 12 35 29](https://slidetodoc.com/presentation_image_h2/5d9e647e2e7d8a1ff30e75a3ad6e7263/image-30.jpg)
Metal Fe Complex [Fe(CN)6]3 - Z X Y EAN 26 -03 12 35 29 -02 08 35 78 -02 08 84 Hexacyanoferrat e (III) ion Cu [Cu(NH 3)4]2+ tetra amine copper (II) ion Pt [Pt(NH 3)4]2+ tetra amine platinum (IV) ion

Bonding in Coordination compounds v Warner was the first to discuss the bonding features in coordination compounds. v There are many theories which can explain the nature of bonding , as well as various properties of coordination compounds. They are: 1) 2) 3) 4) Valence Bond Theory Crystal field theory Ligand field theory Molecular Orbital Theory

Valence Bond Theory v The valence bond theory was developed by Linus Pauling v This theory accounts for coordinate bond formation , due to overlap of vacant hybrid orbital of central metal atom with the filled orbitals of ligands each containing lone pair of electron.

Valence Bond Theory v Central metal ion present in a complex provides a definite number of vacant orbitals , p and d for the formation of coordinate bonds with ligands. v The no. of vacant orbitals provided the central ion is the same as its coordination number. v Each ligand has at least one orbital containing a lone pair of electron. v Vacant orbitals undergo hybridization to form same no. of hybrid orbitals.

Valence Bond Theory v The geometrical shape of the complex ion depends upon hybridization of metal orbital's. v When central metal atom of the complex compound contains one or more unpaired electrons it, exhibits paramagnetic property. v Coordinate bond is stronger, if the overlapping between orbitals is greater.

Valence Bond Theory v If (n-1 )d orbitals are used for hybridization, complexes are called inner complexes. [Co(NH 3)6]3+ If nd orbital's are used for hybridization are called as outer complexes. [Co F 6]3 when strong field ligands like NH 3 and CN- are involved in formation of complexes, they cause pairing of electrons present in metal ions. This process is called spin pairing.

v. Crystal field theory (CFT) 1) It is the interaction between the metal ion and ligands is purely electrostatic. When ligands approach central metal atom / ion the five degenerate d-orbital of the central atom become split up into levels of different energy under the influence of electrostatic field of ligands.

Salient features of crystal field theory 1) In complex central metal atom / ion is surrounded by various atoms / groups of atoms called ligands. 2) Ligands are –vly or neutral molecules posses lone pair of electrons. 3) Both metal ion and ligands act as point charges. 4) Electron of metal atom occupy splited d – orbitals acc. To Hunds rule.

Isomerism in coordination compounds v Isomerism is very common phenomenon in coordination compounds. v Isos meaning equal and meros meaning part. v There are two types of isomerism in coordination compounds: A. Stereo isomerism B. Structural isomerism

v Stereo isomerism : In this type different atom or groups occupy different spatial positions around central metal atom. 1) Geometrical isomerism 2) optical isomerism B)Structural isomerism: It is classified as, 1) Ionization isomerism 2) Linkage isomerism 3) coordination isomerism 4) solvate isomerism

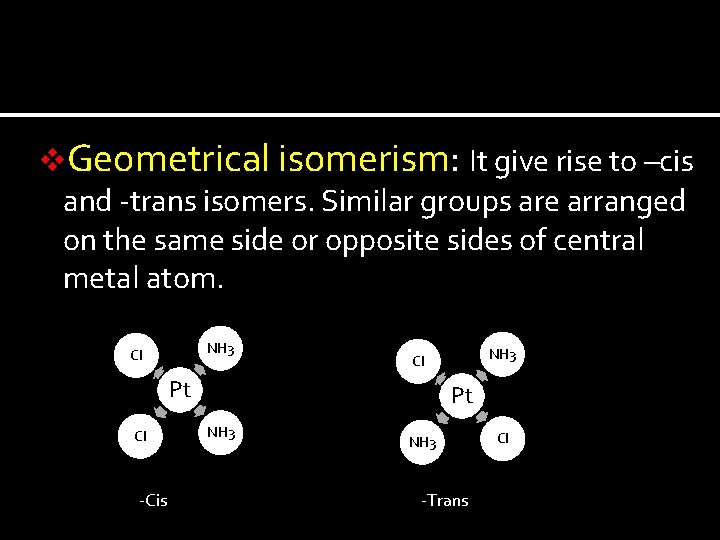
v. Geometrical isomerism: It give rise to –cis and -trans isomers. Similar groups are arranged on the same side or opposite sides of central metal atom. NH 3 Cl Pt Cl -Cis NH 3 Cl Pt NH 3 -Trans Cl

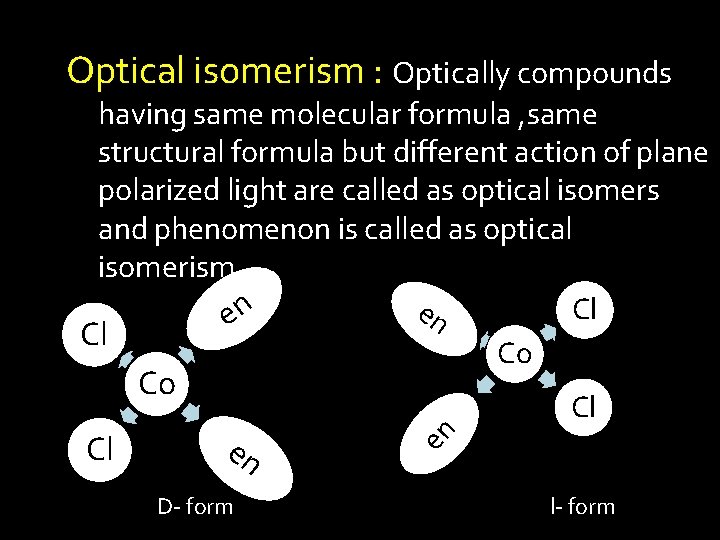
Optical isomerism : Optically compounds having same molecular formula , same structural formula but different action of plane polarized light are called as optical isomers and phenomenon is called as optical isomerism. en Cl Co Co en D- form en Cl Cl l- form

It will give rise to dextro (d) and leavo (l) form. One form rotate the plane of polarized light in clockwise, while other in anticlockwise direction , is called as d- form and l-form. The phenomenon of optical isomerism is quite common in coordination compounds. Complex whose molecules are asymmetric exhibit optical isomerism.

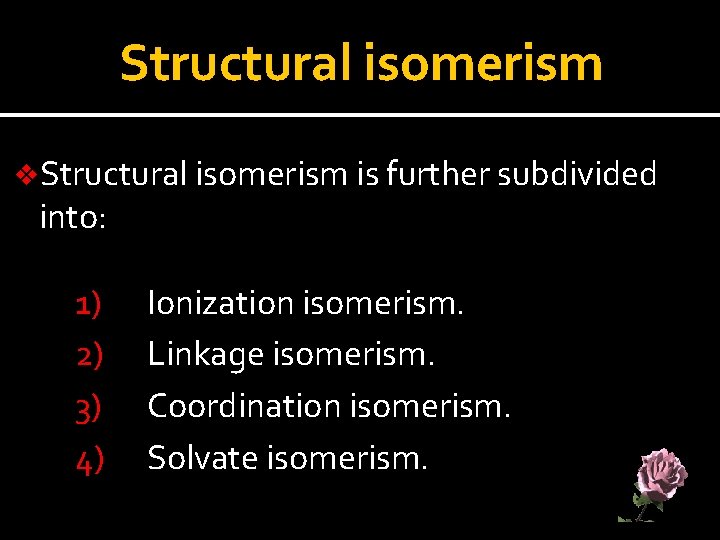
Structural isomerism v. Structural isomerism is further subdivided into: 1) 2) 3) 4) Ionization isomerism. Linkage isomerism. Coordination isomerism. Solvate isomerism.

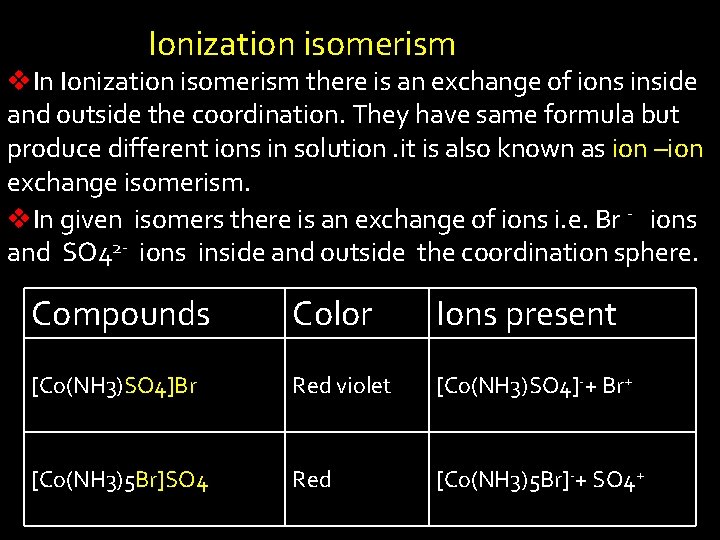
Ionization isomerism v. In Ionization isomerism there is an exchange of ions inside and outside the coordination. They have same formula but produce different ions in solution. it is also known as ion –ion exchange isomerism. v. In given isomers there is an exchange of ions i. e. Br - ions and SO 42 - ions inside and outside the coordination sphere. Compounds Color Ions present [Co(NH 3)SO 4]Br Red violet [Co(NH 3)SO 4]-+ Br+ [Co(NH 3)5 Br]SO 4 Red [Co(NH 3)5 Br]-+ SO 4+

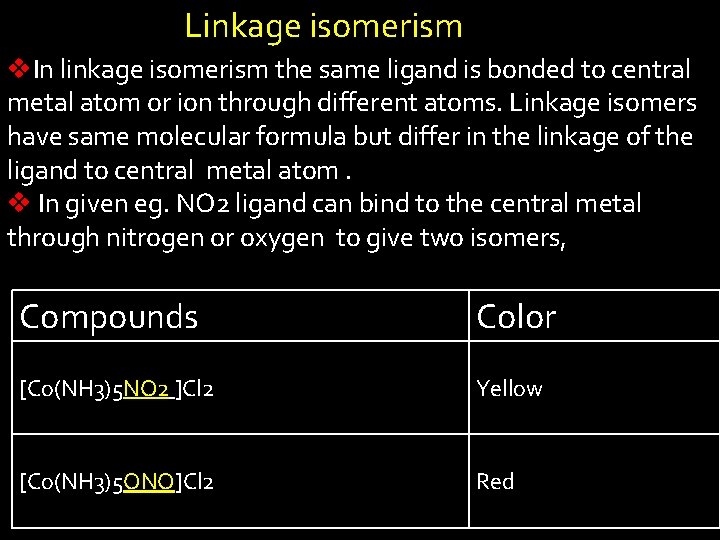
Linkage isomerism v. In linkage isomerism the same ligand is bonded to central metal atom or ion through different atoms. Linkage isomers have same molecular formula but differ in the linkage of the ligand to central metal atom. v In given eg. NO 2 ligand can bind to the central metal through nitrogen or oxygen to give two isomers, Compounds Color [Co(NH 3)5 NO 2 ]Cl 2 Yellow [Co(NH 3)5 ONO]Cl 2 Red

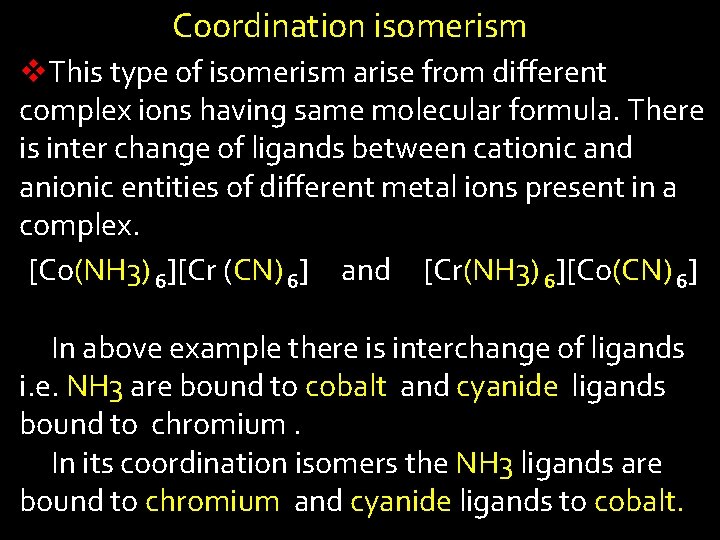
Coordination isomerism v. This type of isomerism arise from different complex ions having same molecular formula. There is inter change of ligands between cationic and anionic entities of different metal ions present in a complex. [Co(NH 3) 6][Cr (CN) 6] and [Cr(NH 3) 6][Co(CN) 6] In above example there is interchange of ligands i. e. NH 3 are bound to cobalt and cyanide ligands bound to chromium. In its coordination isomers the NH 3 ligands are bound to chromium and cyanide ligands to cobalt.

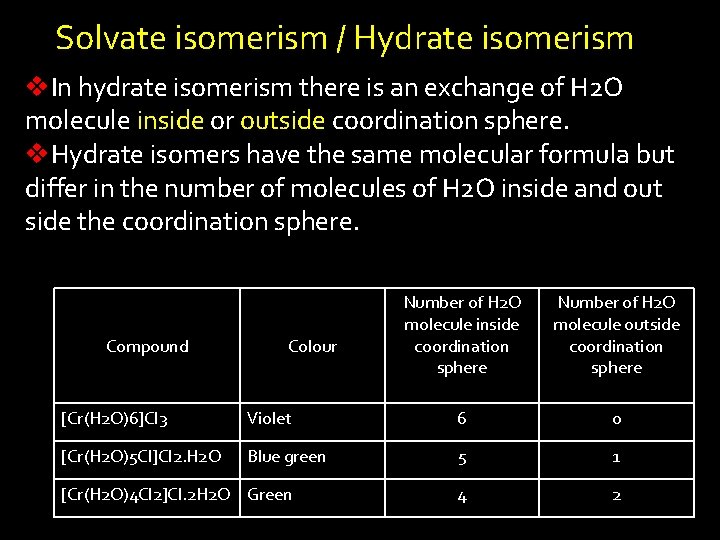
Solvate isomerism / Hydrate isomerism v. In hydrate isomerism there is an exchange of H 2 O molecule inside or outside coordination sphere. v. Hydrate isomers have the same molecular formula but differ in the number of molecules of H 2 O inside and out side the coordination sphere. Compound Colour Number of H 2 O molecule inside coordination sphere Number of H 2 O molecule outside coordination sphere [Cr(H 2 O)6]Cl 3 Violet 6 0 [Cr(H 2 O)5 Cl]Cl 2. H 2 O Blue green 5 1 4 2 [Cr(H 2 O)4 Cl 2]Cl. 2 H 2 O Green

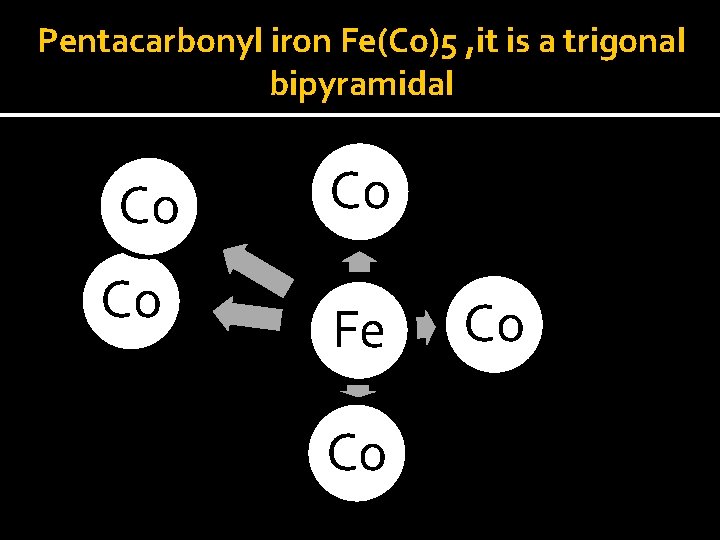
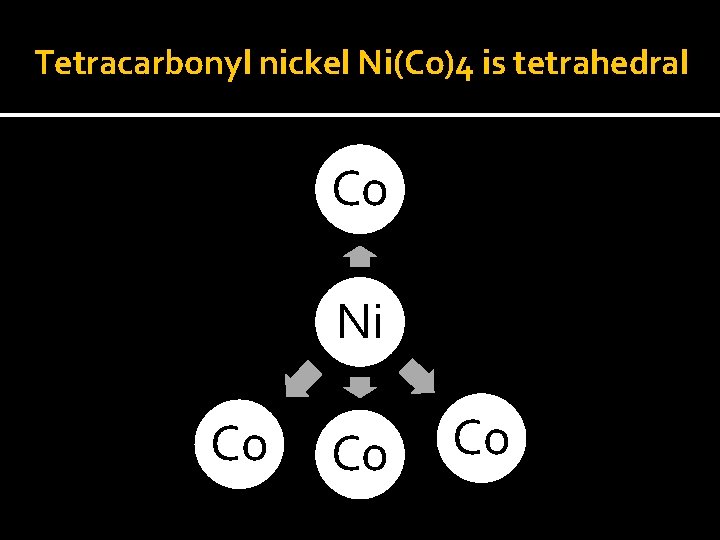
Bonding in Metal Carbnyl v Compound containing carbonyl ligands only are known as homoleptic carbonyl. v Such type of compounds are formed by most of the transition metals. v For: pentacyarbonyl iron (O) is trigonal bypiramidal tetracarbonyl nickel (O) is tetrahedral while hexacarbonyl chromium (O) is octahedral.

Pentacarbonyl iron Fe(Co)5 , it is a trigonal bipyramidal Co Co Co Fe Co Co

Tetracarbonyl nickel Ni(Co)4 is tetrahedral Co Ni Co Co Co

Thank You
 Organic vs inorganic chemistry
Organic vs inorganic chemistry M(aa)3 isomers
M(aa)3 isomers Aquatic food chain
Aquatic food chain Most amazing roads in the world
Most amazing roads in the world Pharmaceutical inorganic chemistry introduction
Pharmaceutical inorganic chemistry introduction Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Is ch4o organic or inorganic
Is ch4o organic or inorganic Inert pair effect
Inert pair effect Fazan rule
Fazan rule Structure of ceramics
Structure of ceramics Correlation diagram in coordination chemistry
Correlation diagram in coordination chemistry Correlation diagram in coordination chemistry
Correlation diagram in coordination chemistry Coordination number
Coordination number Electric field and magnetic field difference
Electric field and magnetic field difference Field dependent vs field independent
Field dependent vs field independent Field dependent vs field independent
Field dependent vs field independent Q factor of capacitor
Q factor of capacitor Waveguide cutoff frequency
Waveguide cutoff frequency Database field types and field properties
Database field types and field properties Field dependent definition
Field dependent definition Magnetic field
Magnetic field Smear layer definizione
Smear layer definizione Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Inorganic plant
Inorganic plant Inorganic vs organic
Inorganic vs organic Importance of organic compounds
Importance of organic compounds Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Organic versus inorganic compounds
Organic versus inorganic compounds Veins are often formed from hot water solutions
Veins are often formed from hot water solutions Organic and inorganic cofactors
Organic and inorganic cofactors Difference between organic and inorganic
Difference between organic and inorganic Classification of coenzyme
Classification of coenzyme Inorganic emulsifying agent is
Inorganic emulsifying agent is Organic and inorganic cofactors
Organic and inorganic cofactors Inorganic catalyst vs enzyme
Inorganic catalyst vs enzyme Phospoholipid
Phospoholipid Binomial nomenclature worksheet
Binomial nomenclature worksheet Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Inorganic mineral definition
Inorganic mineral definition Iupac nomenclature of cycloalkanes
Iupac nomenclature of cycloalkanes Organic vs inorganic compounds
Organic vs inorganic compounds Draw polymeric backbone of silicones and phosphazenes
Draw polymeric backbone of silicones and phosphazenes Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Inorganic geology definition
Inorganic geology definition Calculus subgingival
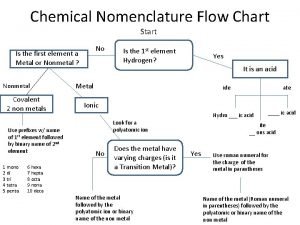
Calculus subgingival Chemistry nomenclature flow chart
Chemistry nomenclature flow chart Gravimetric methods of analysis
Gravimetric methods of analysis Inorganic precipitating agents
Inorganic precipitating agents Difference between organic and inorganic growth
Difference between organic and inorganic growth Inorganic growth disadvantages
Inorganic growth disadvantages Homogeneous minerals
Homogeneous minerals Inorganic non metallic materials examples
Inorganic non metallic materials examples