Coordinate Covalent Bonds 1 Coordinate Covalent Compounds n

Coordinate Covalent Bonds 1
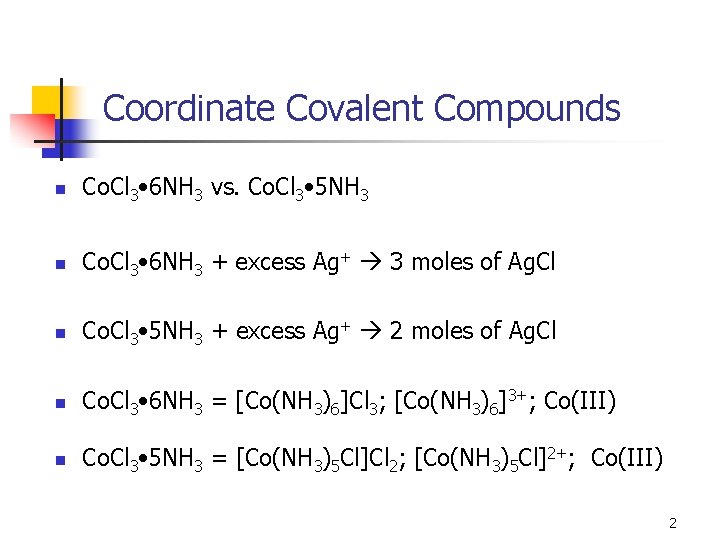
Coordinate Covalent Compounds n Co. Cl 3 • 6 NH 3 vs. Co. Cl 3 • 5 NH 3 n Co. Cl 3 • 6 NH 3 + excess Ag+ 3 moles of Ag. Cl n Co. Cl 3 • 5 NH 3 + excess Ag+ 2 moles of Ag. Cl n Co. Cl 3 • 6 NH 3 = [Co(NH 3)6]Cl 3; [Co(NH 3)6]3+; Co(III) n Co. Cl 3 • 5 NH 3 = [Co(NH 3)5 Cl]Cl 2; [Co(NH 3)5 Cl]2+; Co(III) 2
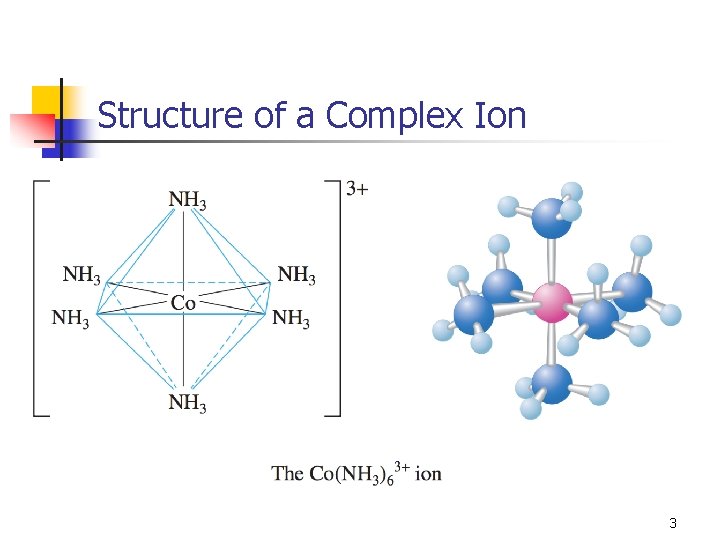
Structure of a Complex Ion 3

Coordination Compounds n Two spheres, or valences: n n Primary: oxidation state (charge of metal ion) Secondary: coordination number (number of ligands, often 2, 4, 6) Counter ions: used to balance charge Ligands n n n Neutral molecule or ion. Lone pair that can be used to bond to the metal ion. Different models are used to describe bonding (as always!). 4
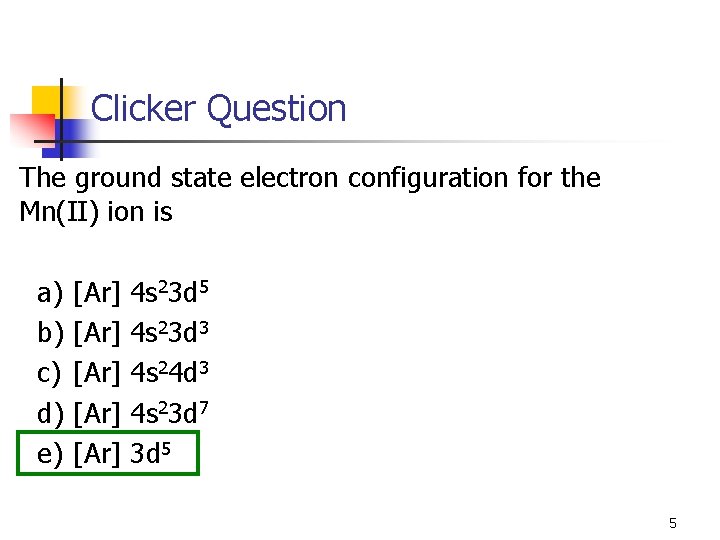
Clicker Question The ground state electron configuration for the Mn(II) ion is a) b) c) d) e) [Ar] [Ar] 4 s 23 d 5 4 s 23 d 3 4 s 24 d 3 4 s 23 d 7 3 d 5 5
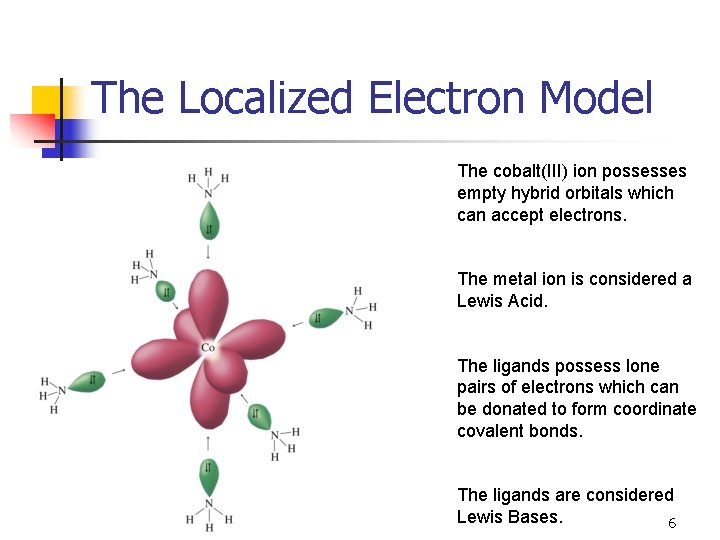
The Localized Electron Model The cobalt(III) ion possesses empty hybrid orbitals which can accept electrons. The metal ion is considered a Lewis Acid. The ligands possess lone pairs of electrons which can be donated to form coordinate covalent bonds. The ligands are considered Lewis Bases. 6
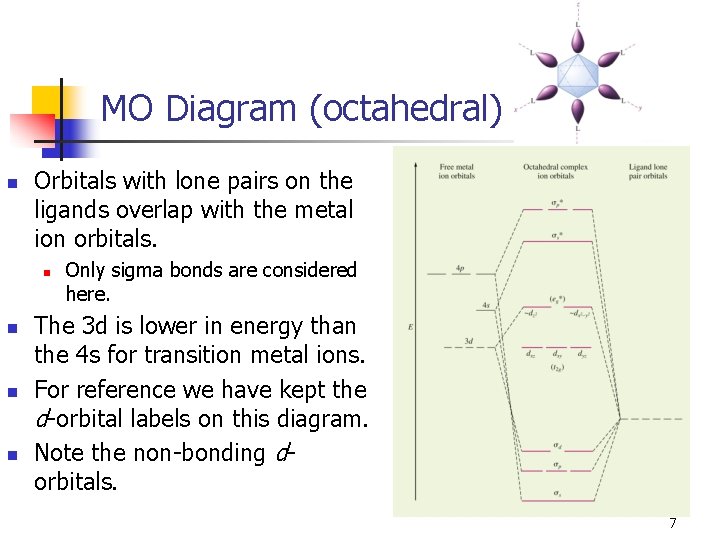
MO Diagram (octahedral) n Orbitals with lone pairs on the ligands overlap with the metal ion orbitals. n n Only sigma bonds are considered here. The 3 d is lower in energy than the 4 s for transition metal ions. For reference we have kept the d-orbital labels on this diagram. Note the non-bonding dorbitals. 7
![MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with](http://slidetodoc.com/presentation_image_h2/46b0c7552e56dfc4e414642ebd0fdac4/image-8.jpg)
MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with 6 lone pairs (octahedral) we always have 12 electrons from the ligands. Thus, the number of electrons in the “dorbital” range of the MO = the number of electrons in the metal ion. 8
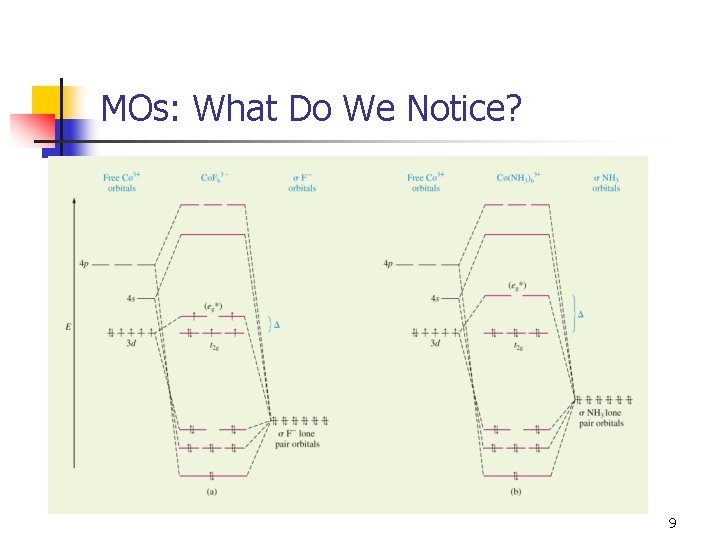
MOs: What Do We Notice? 9
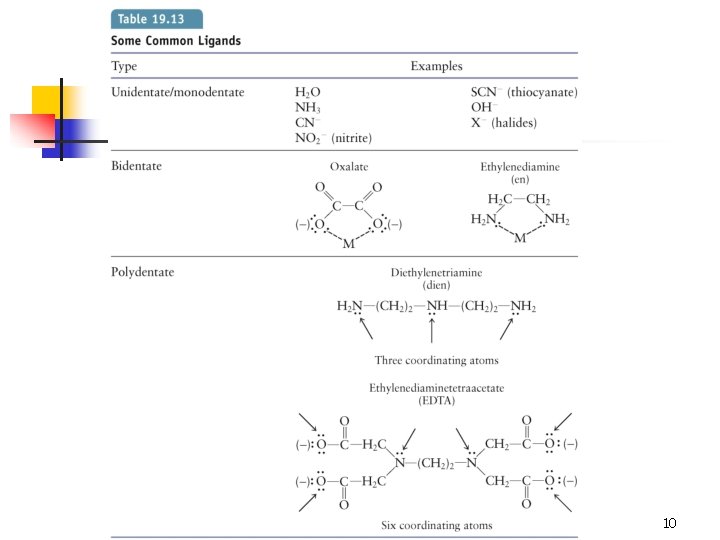
10
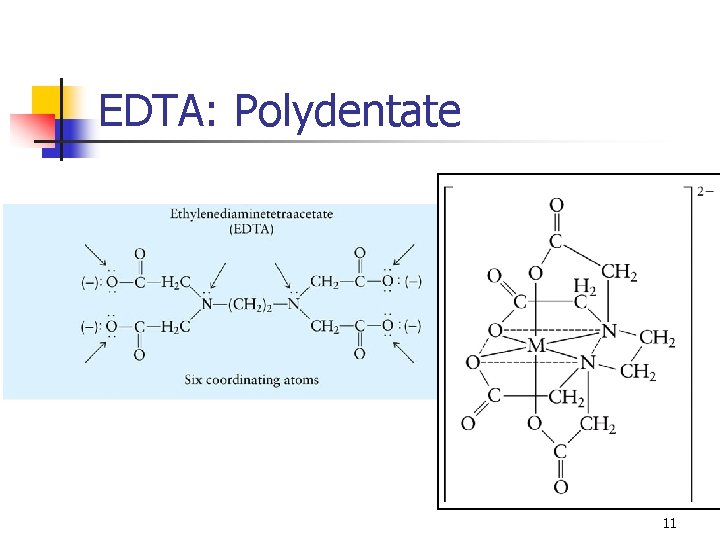
EDTA: Polydentate 11
![Naming Rules: be familiar with them n [Cr(H 2 O)5 Br]Br 2. n n Naming Rules: be familiar with them n [Cr(H 2 O)5 Br]Br 2. n n](http://slidetodoc.com/presentation_image_h2/46b0c7552e56dfc4e414642ebd0fdac4/image-12.jpg)
Naming Rules: be familiar with them n [Cr(H 2 O)5 Br]Br 2. n n n Name will end with “bromide”. Metal ion part of complex cation (+), so just use its name and charge (chromium(III) – why 3+? ) Five (penta) water (aqua) ligands: pentaaqua One bromide ligand: bromo pentaaquabromochromium(III) bromide Na 3[Co(CN)6]. n n n Name will begin with “sodium”. Metal ion part of complex anion (–), so add “ate” and charge (cobaltate(III)) sodium hexacyanocobaltate(III) 12
- Slides: 12