Converting Line Bond Structures to Condensed Formulas LineBond
Converting Line Bond Structures to Condensed Formulas
Line-Bond Structures • In a Line-Bond structure, every atom and every bond is drawn in. • The only thing not shown would be any lone pairs on atoms like oxygen or nitrogen or a halide. • Show all lines and all letters.
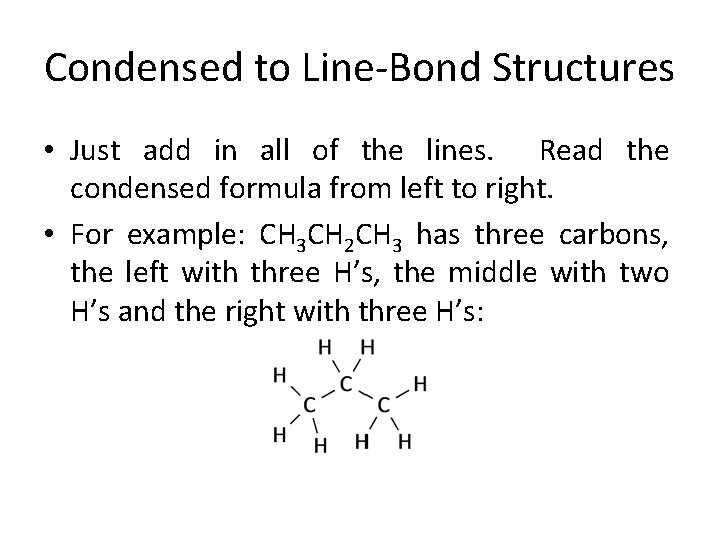
Condensed to Line-Bond Structures • Just add in all of the lines. Read the condensed formula from left to right. • For example: CH 3 CH 2 CH 3 has three carbons, the left with three H’s, the middle with two H’s and the right with three H’s:
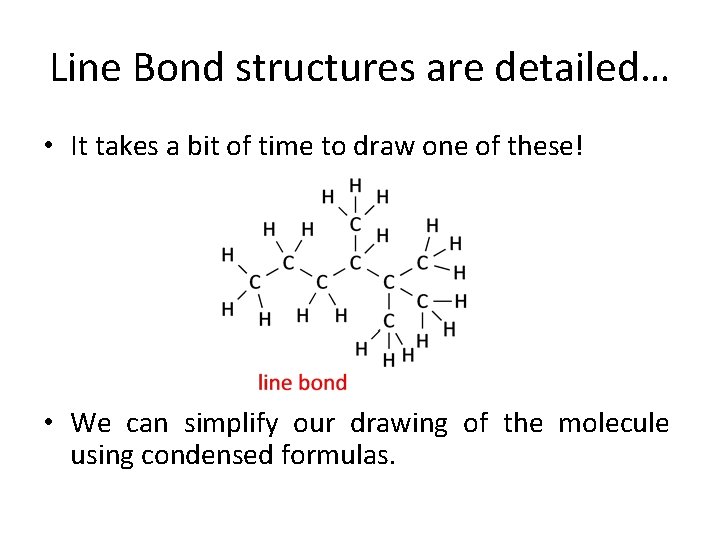
Line Bond structures are detailed… • It takes a bit of time to draw one of these! • We can simplify our drawing of the molecule using condensed formulas.
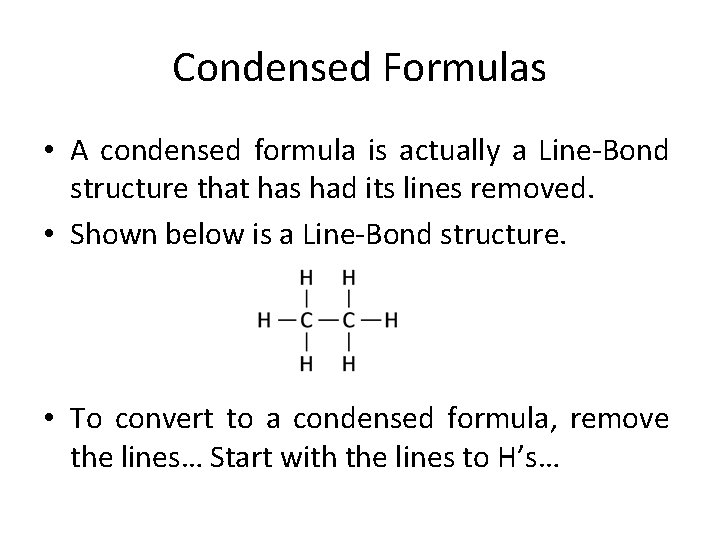
Condensed Formulas • A condensed formula is actually a Line-Bond structure that has had its lines removed. • Shown below is a Line-Bond structure. • To convert to a condensed formula, remove the lines… Start with the lines to H’s…
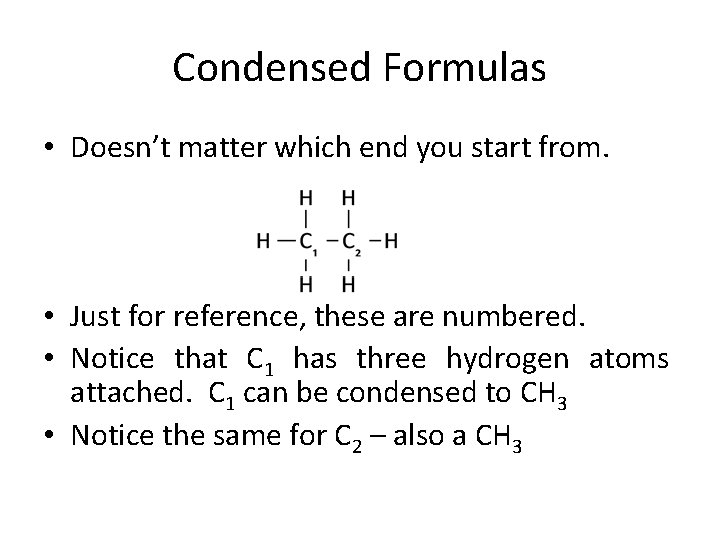
Condensed Formulas • Doesn’t matter which end you start from. • Just for reference, these are numbered. • Notice that C 1 has three hydrogen atoms attached. C 1 can be condensed to CH 3 • Notice the same for C 2 – also a CH 3
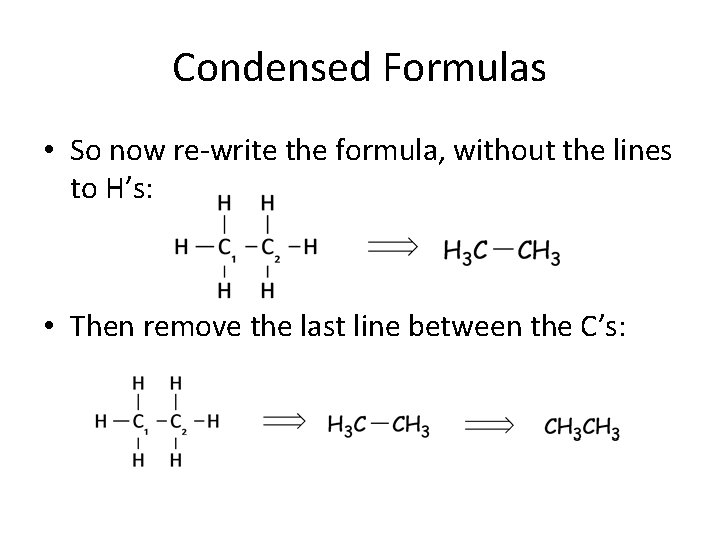
Condensed Formulas • So now re-write the formula, without the lines to H’s: • Then remove the last line between the C’s:
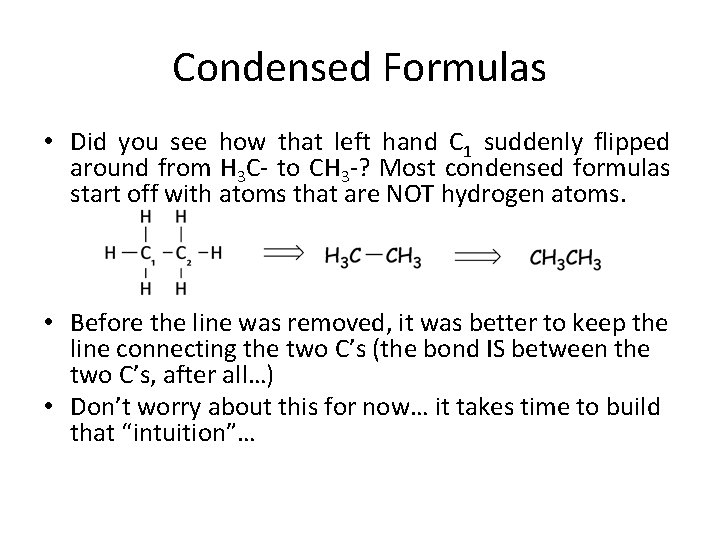
Condensed Formulas • Did you see how that left hand C 1 suddenly flipped around from H 3 C- to CH 3 -? Most condensed formulas start off with atoms that are NOT hydrogen atoms. • Before the line was removed, it was better to keep the line connecting the two C’s (the bond IS between the two C’s, after all…) • Don’t worry about this for now… it takes time to build that “intuition”…
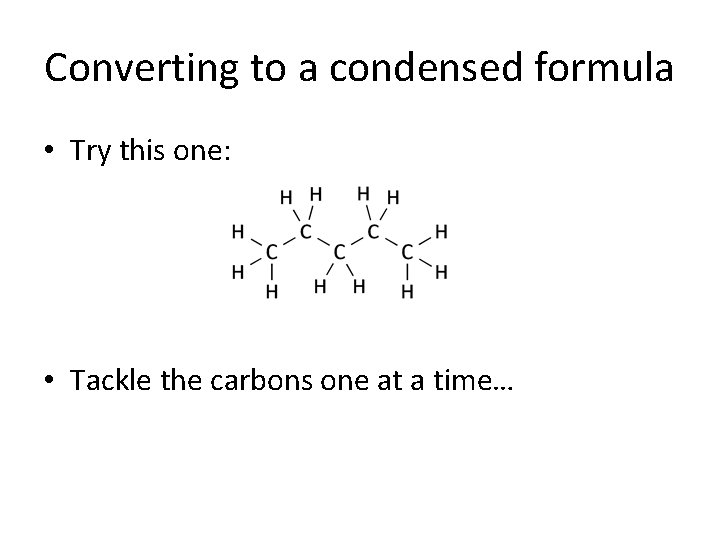
Converting to a condensed formula • Try this one: • Tackle the carbons one at a time…
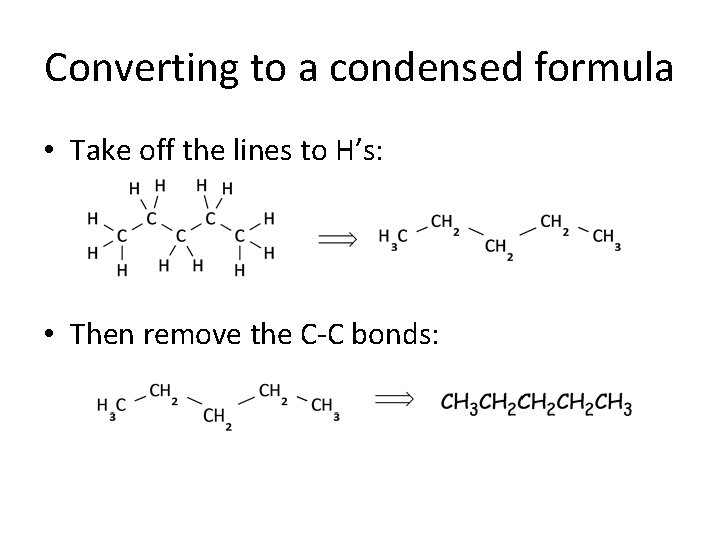
Converting to a condensed formula • Take off the lines to H’s: • Then remove the C-C bonds:
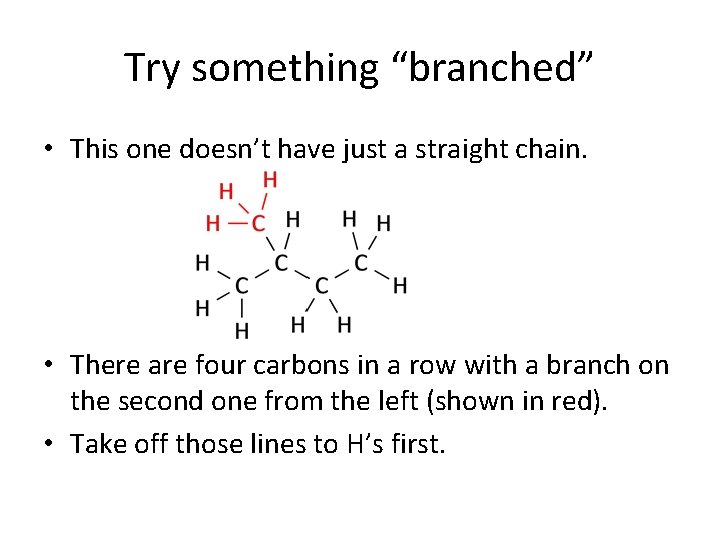
Try something “branched” • This one doesn’t have just a straight chain. • There are four carbons in a row with a branch on the second one from the left (shown in red). • Take off those lines to H’s first.
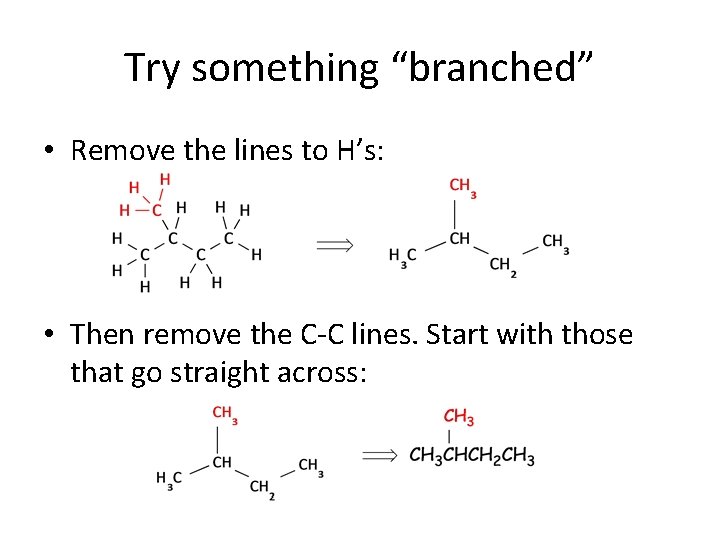
Try something “branched” • Remove the lines to H’s: • Then remove the C-C lines. Start with those that go straight across:
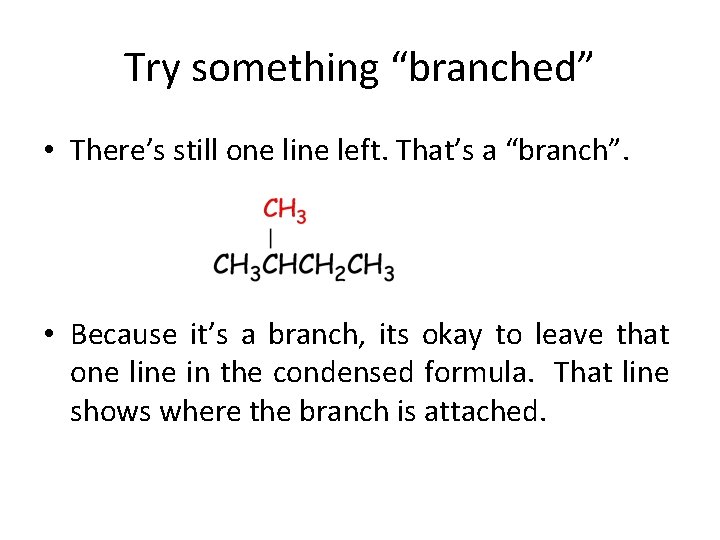
Try something “branched” • There’s still one line left. That’s a “branch”. • Because it’s a branch, its okay to leave that one line in the condensed formula. That line shows where the branch is attached.
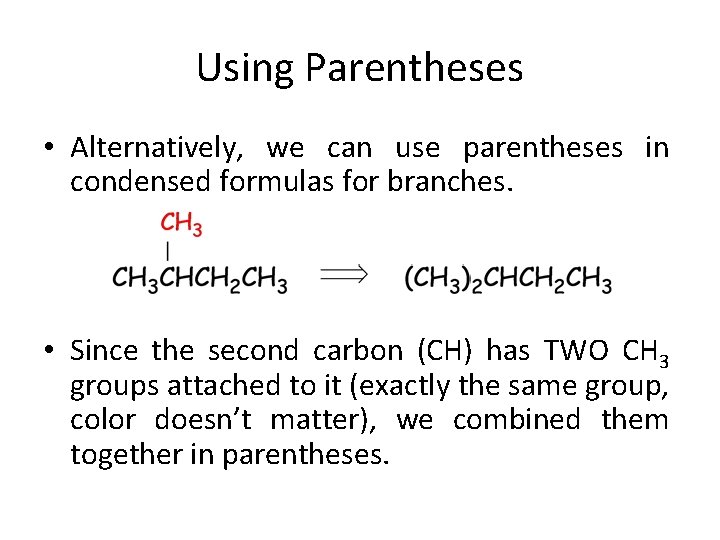
Using Parentheses • Alternatively, we can use parentheses in condensed formulas for branches. • Since the second carbon (CH) has TWO CH 3 groups attached to it (exactly the same group, color doesn’t matter), we combined them together in parentheses.
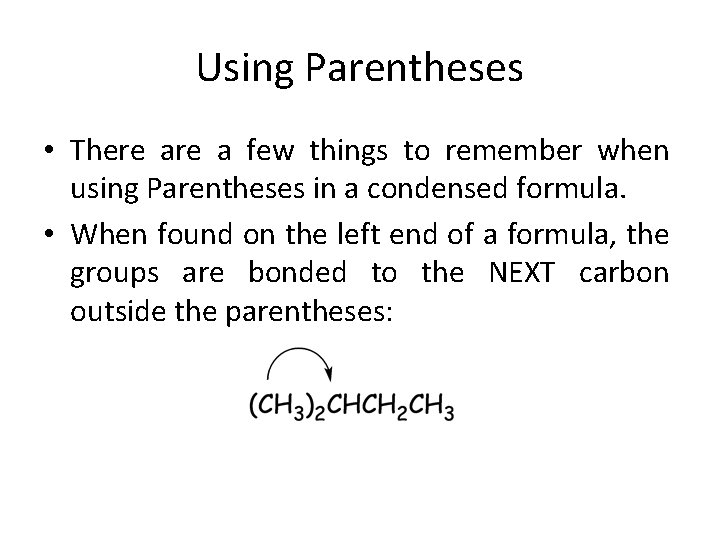
Using Parentheses • There a few things to remember when using Parentheses in a condensed formula. • When found on the left end of a formula, the groups are bonded to the NEXT carbon outside the parentheses:
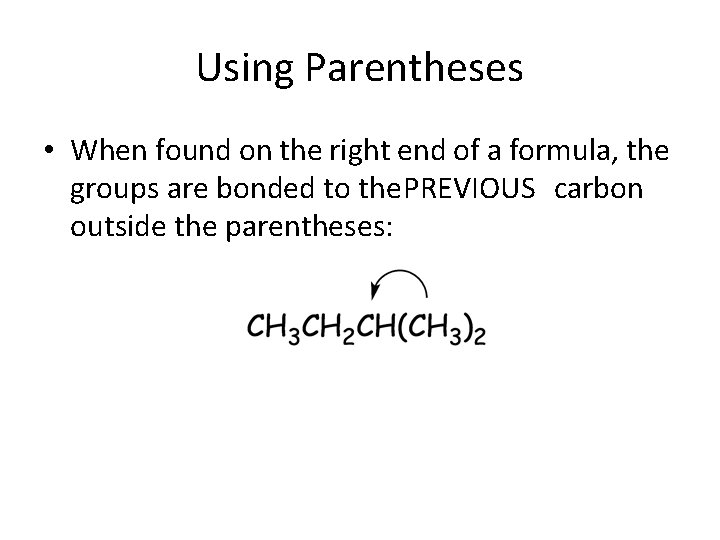
Using Parentheses • When found on the right end of a formula, the groups are bonded to the. PREVIOUS carbon outside the parentheses:
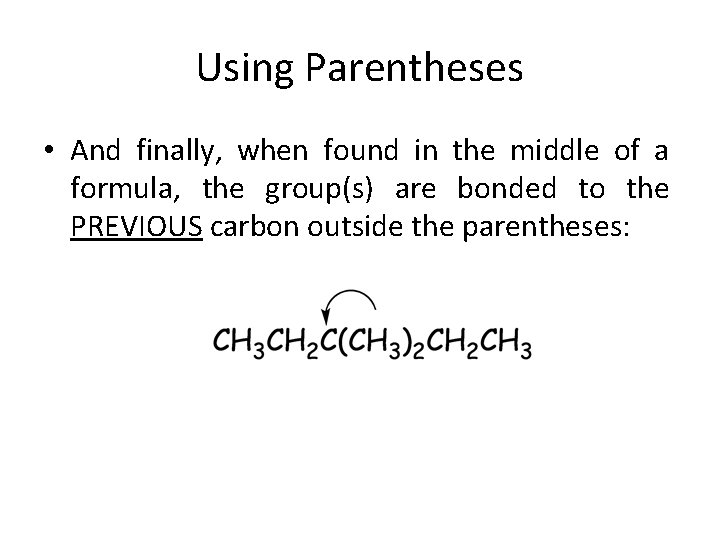
Using Parentheses • And finally, when found in the middle of a formula, the group(s) are bonded to the PREVIOUS carbon outside the parentheses:
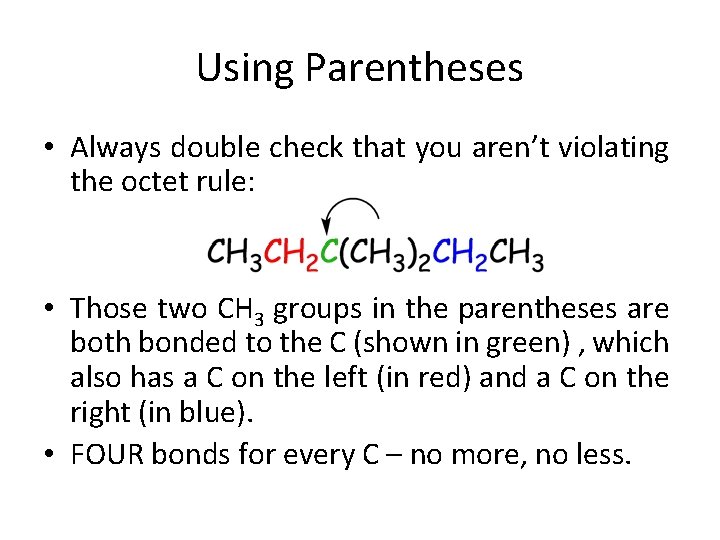
Using Parentheses • Always double check that you aren’t violating the octet rule: • Those two CH 3 groups in the parentheses are both bonded to the C (shown in green) , which also has a C on the left (in red) and a C on the right (in blue). • FOUR bonds for every C – no more, no less.
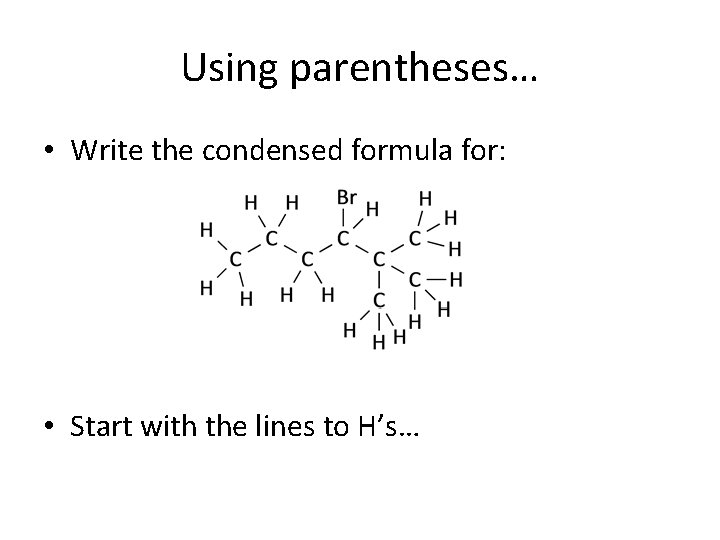
Using parentheses… • Write the condensed formula for: • Start with the lines to H’s…
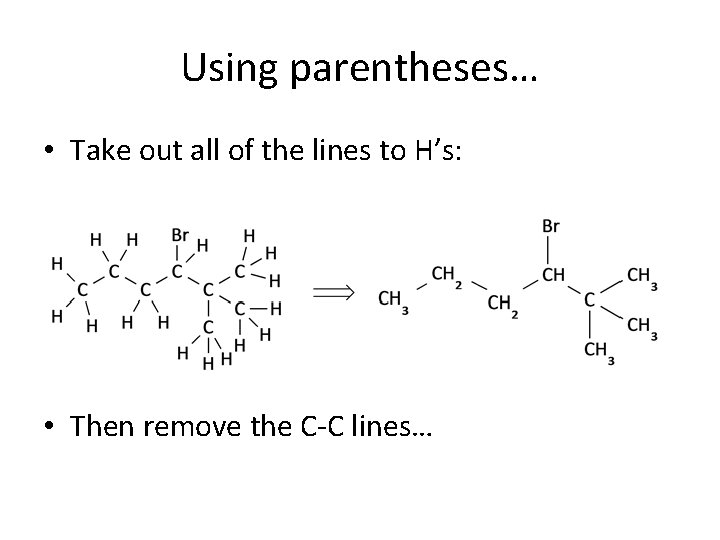
Using parentheses… • Take out all of the lines to H’s: • Then remove the C-C lines…
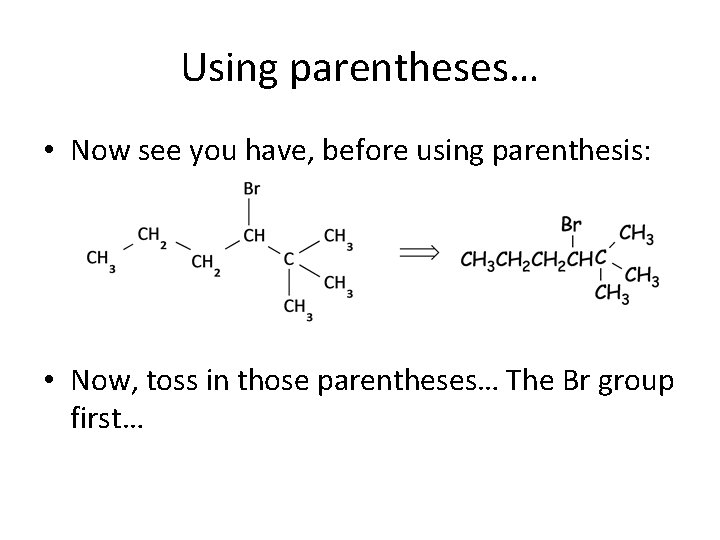
Using parentheses… • Now see you have, before using parenthesis: • Now, toss in those parentheses… The Br group first…
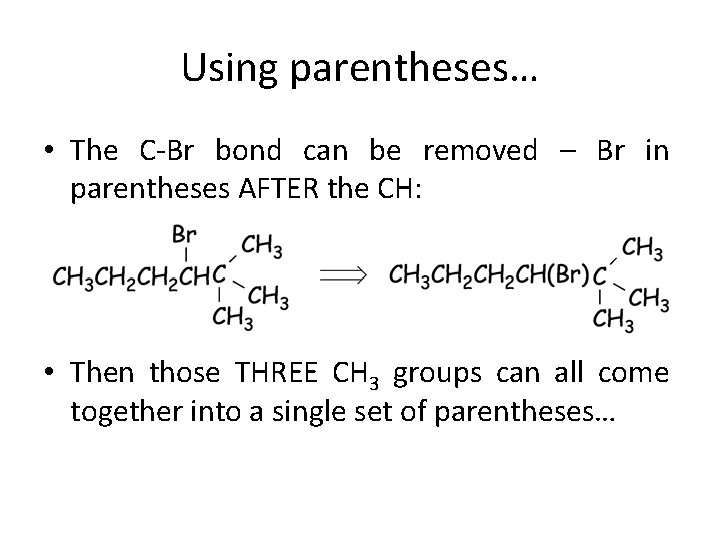
Using parentheses… • The C-Br bond can be removed – Br in parentheses AFTER the CH: • Then those THREE CH 3 groups can all come together into a single set of parentheses…
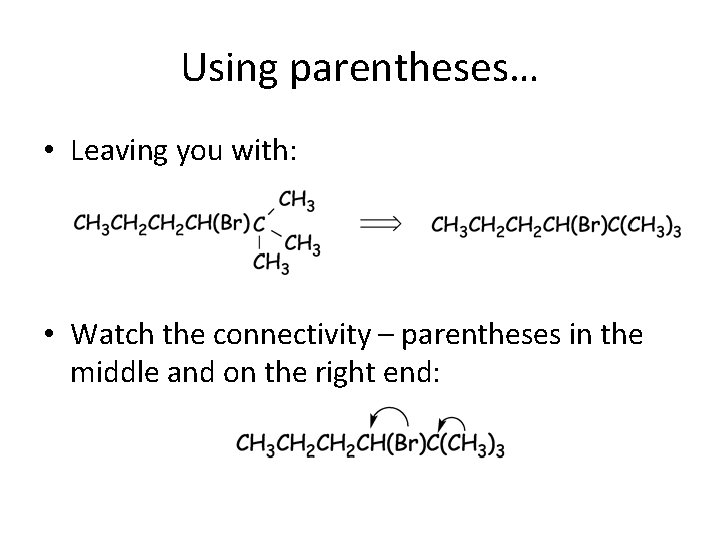
Using parentheses… • Leaving you with: • Watch the connectivity – parentheses in the middle and on the right end:
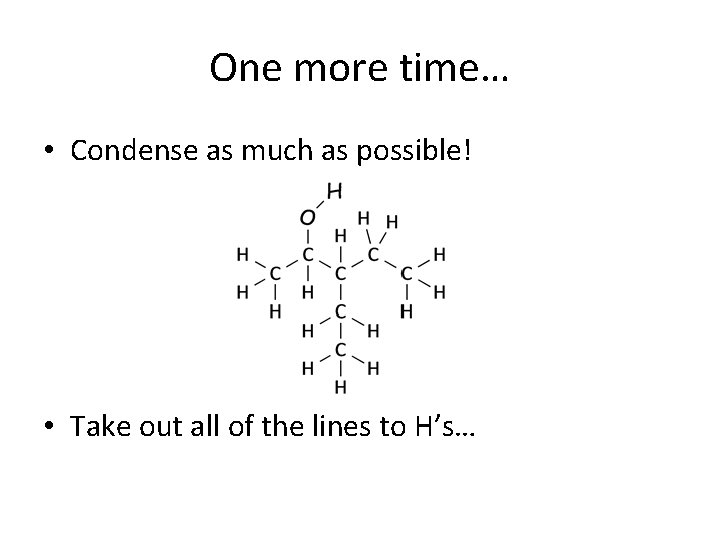
One more time… • Condense as much as possible! • Take out all of the lines to H’s…
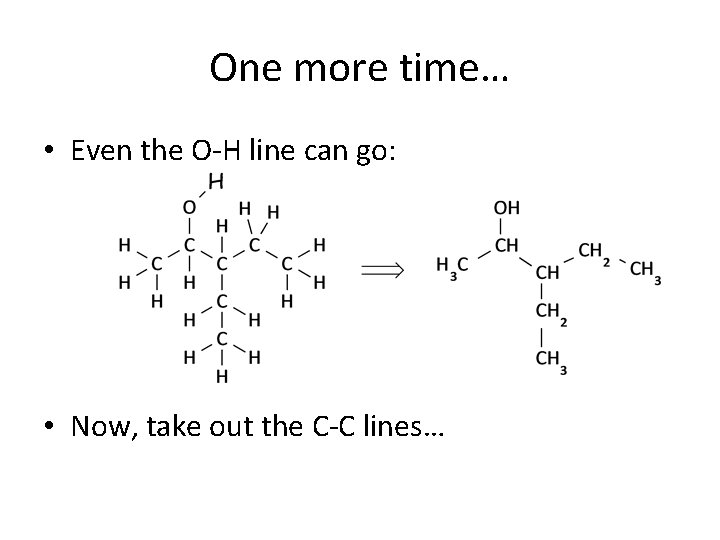
One more time… • Even the O-H line can go: • Now, take out the C-C lines…
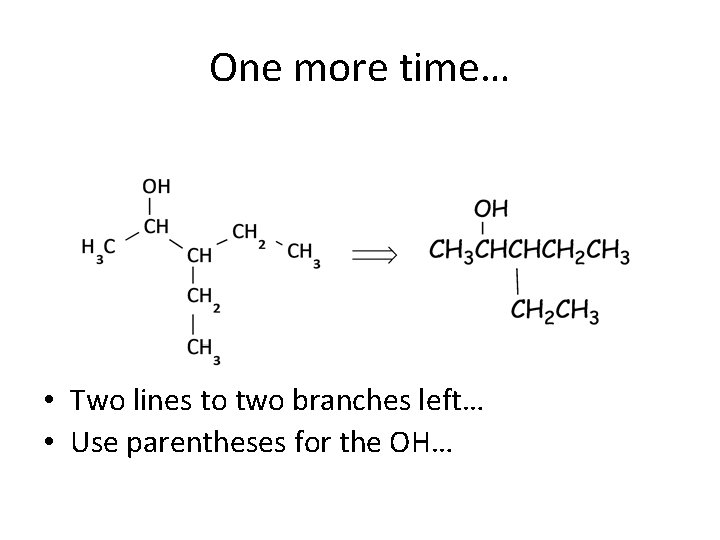
One more time… • Two lines to two branches left… • Use parentheses for the OH…
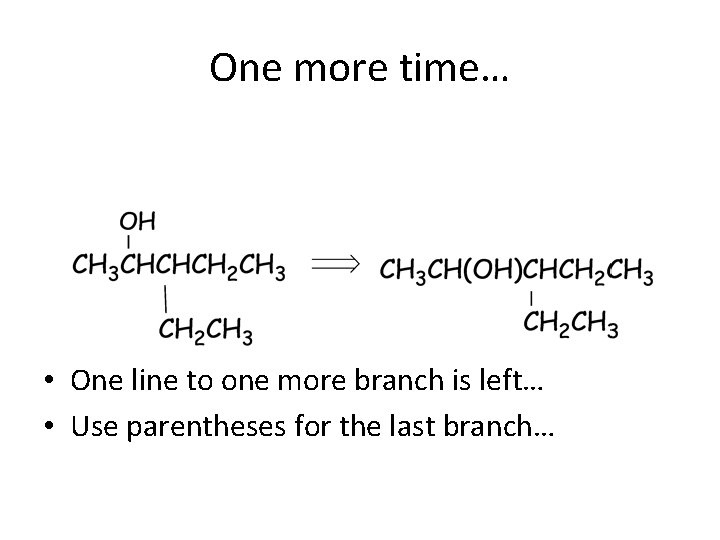
One more time… • One line to one more branch is left… • Use parentheses for the last branch…
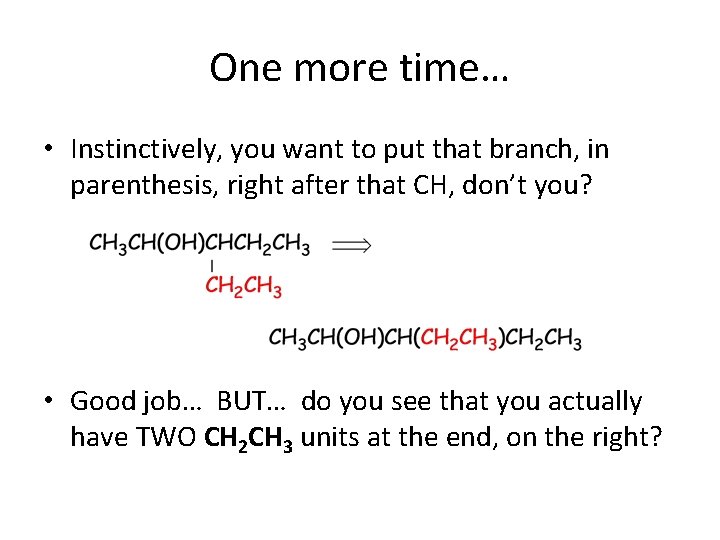
One more time… • Instinctively, you want to put that branch, in parenthesis, right after that CH, don’t you? • Good job… BUT… do you see that you actually have TWO CH 2 CH 3 units at the end, on the right?
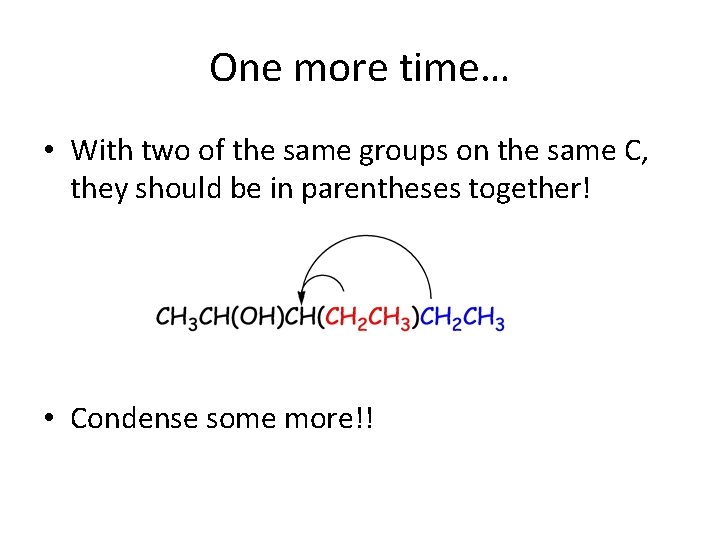
One more time… • With two of the same groups on the same C, they should be in parentheses together! • Condense some more!!
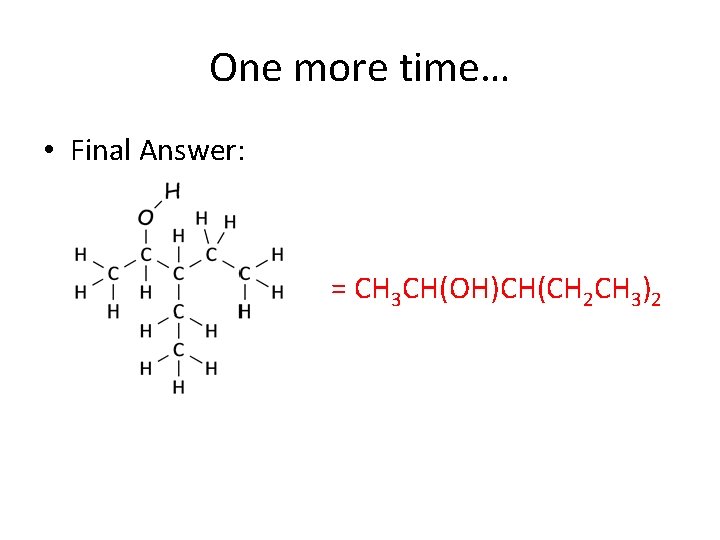
One more time… • Final Answer: = CH 3 CH(OH)CH(CH 2 CH 3)2

Condensed, Line-Bond, Condensed…. • Practice makes perfect… and then you can move on to Skeletal Structures… • Go find the Power. Point “Drawing Skeletal (Zig. Zag) Structures” to head to the next level…
- Slides: 31